All rights reserved. No part of this book may be reprinted or reproduced or utilised in any form or by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying and recording, or in any information storage or retrieval system, without permission in writing from the publishers. Enquiries in this regard should be directed to the British Psychological Society.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Mental Health (UK). Social Anxiety Disorder: Recognition, Assessment and Treatment. Leicester (UK): British Psychological Society (UK); 2013. (NICE Clinical Guidelines, No. 159.)
6.1. INTRODUCTION
Social anxiety disorder was formally recognised as a separate phobic disorder in the mid 1960s (Marks & Gelder, 1965), but, as described in Chapter 2, the formal recognition of the disorder has not been widespread, with over half of people with a social anxiety disorder never seeking treatment. Many of those who seek treatment may not have their disorder recognised and, as a consequence, may be offered inappropriate or suboptimal treatment. The past 20 years have seen the development of an evidence base of effective interventions, but these have not always been available (Layard et al., 2006) even when the need for treatment has been recognised. This is a source of real concern because social anxiety disorder can have lifelong and disabling consequences.
This chapter is concerned primarily with the evaluation of psychological and pharmacological interventions, but also considers other physical interventions including botulinum toxin and thoracic sympathectomy.
6.2. CURRENT PRACTICE
6.2.1. Pharmacological interventions
Previous reviews suggest there is evidence that pharmacotherapy may be efficacious for the treatment of social anxiety disorder (Blanco et al., 2012) and several drugs are licensed in the UK for the treatment of the disorder (escitalopram, moclobemide, paroxetine, sertraline, and venlafaxine). Other SSRIs have also been evaluated in the treatment of social anxiety disorder. Monoamine oxidase inhibitors (MAOIs), principally phenelzine and moclobemide, have been used for the treatment of social anxiety disorder as have the anticonvulsants gabapentin and pregabalin. Benzodiazepines have also been used, but their long-term use is actively discouraged. Beta-antagonists such as atenolol or propranolol have often been used to treat specific symptoms such as tremor. However, a number of factors significantly limit the current use of drugs in the treatment of social anxiety disorder. These include: under-recognition or misdiagnosis, which may be related to the masking of the social anxiety disorder by comorbid problems such as depression or alcohol misuse; an unwillingness on the part of many people with social anxiety disorder to take medication for what they perceive to be a personal failing rather than a mental disorder; concerns about side effects; and lack of knowledge on the part of some prescribers about the potential value and the means to provide the necessary support to obtain an optimal outcome from the use of medication. In relation to this latter factor, there is evidence to support the role of prescribers in encouraging graduated exposure in enhancing the efficacy of drugs, and this may occur in good practices. Further issues hampering the effective use of drugs in the treatment of SSRIs include uncertainty about the duration of treatment or their use in combination with psychological interventions (although it should be noted perhaps 20 to 30% of participants in trials of psychological interventions take medication throughout the trials). All this means that current pharmacological treatment for many people with social anxiety disorder is often suboptimal outside a few specialist tertiary treatment centres and for the few people with social anxiety disorder who are offered treatment by specialists in secondary care mental health services.
6.2.2. Psychological interventions
The past 30 years have seen a very significant expansion in the range and availability of psychological interventions for the treatment of social anxiety disorder. Early evidence-based interventions focused on systematic desensitisation and flooding. These were replaced by treatments that involved confronting the feared stimulus in real life (Marks, 1975). Much current evidence-based practice for the treatment of social anxiety disorder has been influenced by this approach. This is most obviously seen in exposure in vivo therapy (see Chapter 2) and the development of a range of cognitive and cognitive behavioural interventions, for which there is substantial evidence for the treatment of social anxiety disorder and other anxiety disorders. These interventions can be provided either individually or in groups, although there has been less emphasis on group CBT treatments in the UK when compared with the US or Australia. Other psychological interventions such as IPT, counselling and psychodynamic therapy have also been used for the treatment of social anxiety disorder. Although many service users may prefer psychological interventions, availability in the NHS has been, until recently, very limited. In 2007 the English Department of Health established the Improving Access to Psychological Therapies (IAPT) programme (Clark et al., 2009), which aimed to very significantly increase the availability of evidence-based psychological interventions so that the outcomes obtained in clinical trials could be provided throughout the NHS. There has been impressive progress over the past 5 years (Clark, 2011) with over 4,000 additional therapists trained and by 2015 an additional 900,000 people projected to be receiving treatment.
6.3. DEFINITIONS AND AIMS OF INTERVENTIONS
6.3.1. Pharmacological interventions
There are three main classes of drug that are used in treating social anxiety disorder: (1) antidepressants, (2) benzodiazepines, and (3) anticonvulsants. Other drugs such as beta-antagonists, antipsychotics, St John's wort and cognitive enhancers have also been used in the treatment of social anxiety disorder.
Antidepressants
The efficacy of antidepressants is thought to be linked to increases in serotonin and possibly dopamine concentrations in the brain.
Selective serotonin reuptake inhibitors and serotonin and noradrenaline reuptake inhibitors
SSRIs act by increasing serotonin concentration in the brain and have been used in social anxiety disorder as well as other anxiety disorders. The only SNRI that has been studied extensively is venlafaxine and it is possible that its effect in social anxiety disorder is mediated solely through changes in serotonin at usually prescribed doses.
Monoamine oxidase inhibitors
MAOIs inhibit the breakdown of noradrenaline, dopamine, serotonin, melatonin, tyramine and phenylethylamine. This effect is not limited to the brain and affects other parts of the body rich in MAO (for example, the gut). The inhibition of MAO may result in a potentially dangerous interaction with tyramine-containing foods and with some medications leading to episodes of dangerously high blood pressure. This risk is much reduced with moclobemide because it is ‘reversible’.
Benzodiazepines
Benzodiazepines augment the effect of gamma-Aminobutyric acid, the main inhibitory neurotransmitter in the brain. The use of benzodiazepines is restricted because of potential tolerance and dependence that can develop when administered for prolonged periods of time.
Anticonvulsants
Anticonvulsants, specifically alpha2delta calcium-gated channel blockers, reduce neuronal excitability and are also used in the treatment of neuropathic pain. Their mechanism of action is not currently understood.
Antipsychotics
Antipsychotics are a class of drugs that act on dopamine receptors and are widely used to treat schizophrenia, bipolar disorder and other serious mental illnesses. They have also been used to treat depression and other disorders including anxiety disorders. They have a wide range of side effects including movement disorders, weight gain and sedation.
Cognitive enhancers
The cognitive enhancer D-cycloserine is a partial agonist of the N-methyl-D-aspartate-associated glycine site. It has been tested as an adjunct to psychological interventions in clinical trials.
6.3.2. Psychological interventions
The following section contains definitions of the commonly used psychological interventions included in the review in this chapter. Fuller descriptions can be found in Chapter 2.
Exposure in vivo
Exposure encourages the person to repeatedly confront situations they fear. It is sometimes done following a hierarchy from least to most feared situations. Repeated exposure may lead to habituation. Exposure may also be designed to challenge and to disconfirm unrealistic and maladaptive beliefs. Exposure exercises involve confrontation with real life (‘in vivo’) social situations through role plays and out-of-office exercises in therapy sessions, and through systematic homework assignments.
Applied relaxation
Applied relaxation is a specialised form of relaxation training that aims to teach people how to relax in common social situations. Starting with training in traditional progressive muscle relaxation, the treatment takes individuals through a series of steps that enables them to relax on cue in everyday situations. The final stage of the treatment involves intensive practice in using the relaxation techniques in real life social situations.
Social skills training
Social skills training is based on the assumption that people are anxious in social situations partly because they are uncertain about how to behave. The treatment involves systematic training in non-verbal social skills (for example, increased eye contact, friendly attentive posture, and so on) and verbal social skills (for example, how to start a conversation, how to give others positive feedback, how to ask questions that promote conversation, and so on). The skills that are identified with the therapist are usually repeatedly practiced through role plays in therapy sessions as well as in homework assignments. As with exposure, social skills training may work through habituation or by disconfirming negative beliefs.
Cognitive behavioural therapy
CBT typically involves exposure in vivo and cognitive restructuring along with training in relaxation techniques and/or social and conversational skills training. CBT can be delivered in either individual or group format.
Cognitive therapy
CT is a variant of CBT and focuses on: (a) the negative beliefs that individuals with social anxiety disorder hold about themselves and social interactions; (b) negative self-imagery; and (c) the problematic cognitive and behavioural processes that occur in social situations (self-focused attention, safety behaviours, and so on). The treatment is usually delivered on an individual basis. However, there is a need for the therapist to be able to call on other people to participate in within-session role plays. This is easier to do if sessions are 90 minutes, rather than the usual 50 minutes.
Interpersonal psychotherapy
Interpersonal psychotherapy (IPT) was originally developed as a treatment for depression. There are three phases to the treatment. In the first phase, the person is encouraged to see social anxiety disorder as an illness that has to be coped with rather than as a sign of weakness or deficiency. In the second phase, the therapist works with the person to address specific interpersonal problems particularly in the areas of role transition and role disputes, but sometimes also grief. Role plays encouraging the expression of feelings and accurate communication are emphasised. People are also encouraged to build a social network comprising close and trusting relationships. In the last phase, the therapist and the service user review progress, address ending of the therapeutic relationship, and prepare for challenging situations and experiences in the future.
Short-term psychodynamic psychotherapy
Short-term psychodynamic psychotherapy sees the symptoms of social anxiety disorder as the result of core relationship conflicts predominately based on early experience. Therapy aims to help the person become aware of the link between conflicts and symptoms. The therapeutic relationship is a central vehicle for insight and change.
Mindfulness training
Mindfulness training encourages individuals to gain psychological distance from their worries and negative emotions, seeing them as an observer would see them, rather than being engrossed in them. There are two varieties of mindfulness training considered in this guideline: mindfulness-based stress reduction and mindfulness-based cognitive therapy.
Treatment starts with general education about stress and social anxiety. Participants then attend weekly groups in which they are taught meditation techniques. Formal meditation practice for at least 30 minutes per day using audiotapes for guidance is also encouraged.
Self-help interventions
Self-help interventions are psychological interventions typically based on cognitive behavioural principles that seek to equip people with strategies and techniques to begin to overcome and manage their psychological difficulties. Self-help usually provides information in the form of books or other written materials that include psychoeducation about the problem and describe techniques to overcome it. Although computerised interventions have the potential to be interactive and individualised, those that have been tested in clinical trials for people with social anxiety are, for the most part, relatively fixed programmes. In ‘pure’, unsupported self-help, only the written materials are used; in supported self-help, a therapist or alternatively a computer-based system (stand alone or web based) assists the service user in using the materials. Supported self-help typically includes 2 to 3 hours of assistance.
Supportive therapy
Supportive therapy uses techniques that aim to enable patients to feel comfortable in discussing their personal experiences in the context of the patient-therapist relationship.
Cognitive bias modification
Cognitive bias modification is a computerised intervention that aims to reduce attention towards threatening stimuli. The most common programs use modified dot-probe tasks in which participants see numerous (sometimes hundreds of) presentations of written or facial stimuli and are asked to make quick decisions based on what has been seen. For example, some tasks present written stimuli with two possible interpretations, one threatening and one benign; participants select one and receive positive reinforcement when they bias towards neutral stimuli. These interventions require limited therapist input and, until recently, these programs were used only to study psychological processes.
Exercise
Exercise is a physical activity that is planned, structured and repetitive, and aims to improve or maintain physical fitness. It may improve anxiety levels and mood generally, provide opportunities to interact with others or function as a form of exposure (for example, for people with a fear of blushing or sweating) and for this reason is classed as a psychological intervention.
6.3.3. Physical interventions
Botulinum toxin
Botulinum toxin is a neurotoxin produced by the bacterium clostridium botulinum, which can cause botulism, a serious and life-threatening illness. In the 1960s it was developed as a medical treatment for conditions such as blepharospasm and strabismus. Medical use of the toxin has increased substantially in the past 20 years and has a wide range of uses including the treatment of excessive sweating (hyperhidrosis) in specific parts of the body through localised injections.
Thoracic sympathectomy
Thoracic sympathectomy is used to treat excessive sweating (hyperhidrosis) and has also been used to help treat extreme facial flushing. It involves cutting the sympathetic nerve (through a small incision in the chest), which switches off sweating and blushing in specific parts of the body.
6.4. CLINICAL REVIEW PROTOCOL
The review protocol, including the review questions, information about the databases searched and the eligibility criteria used for this section of the guideline, can be found in Table 9 (further information about the search strategy can be found in Appendix 6). Parts of these questions were addressed in Cochrane reviews (Cabrera et al., 2012; Depping et al., 2010; Norton, 2012; Stein et al., 2000; Wei & Higgins, 2013; Wiltink et al., 2011), but the searches were up to 7 years old and all needed to be updated.
Table 9
Clinical review protocol for the review of interventions in adults with social anxiety disorder.
6.5. OVERVIEW OF STUDIES CONSIDERED AND CLINICAL EVIDENCE
A systematic review was conducted to identify RCTs of interventions for adults with social anxiety disorder. The search identified 142 relevant RCTs published between 1988 and 2013. Of these, 100 reported continuous outcomes and compared interventions that the GDG considered could be used as primary treatments for people with social anxiety disorder. Studies of short-term interventions (atenolol) and pharmacological interventions that would not be used in clinical practice (noradrenaline reuptake inhibitors, neurokinin-1 antagonists and St John's wort) were excluded (Furmark et al., 2005; Kobak et al., 2005; Liebowitz et al., 1990; Ravindran et al., 2009). The included trials were used in a NMA comparing symptoms of social anxiety following acute treatment, which included results from approximately 13,097 participants. Of the 100 included trials, 25 reported recovery (loss of diagnosis), which was also included in the model (see Chapter 3 for the method and Appendices 12 and 13 for a list of the studies by intervention and the study characteristics).
Trials of particular subgroups (for example, adults with comorbid substance misuse) and trials of different phases of the disorder (for example, relapse prevention studies) were analysed separately. For interventions that the GDG considered recommending on the basis of the NMA, secondary outcomes (depression, quality of life, anxiety-related disability and withdrawal) and controlled follow-up compared with waitlist and placebo are reported where possible. Analyses of secondary outcomes were not conducted for interventions that the GDG decided not to recommend based on the primary analysis. Uncontrolled follow-up data and other comparisons (for example, between two active interventions) were not analysed. Several comparisons did not connect to the network (that is, neither intervention was included), and these were considered in separate pairwise analyses (see Chapter 3 for the method). Relapse prevention studies (that is, people who responded to acute pharmacotherapy and were randomised to continuation therapy or placebo) were also analysed separately. Studies that were excluded from the analysis and reasons for exclusion can be found in Appendix 25, including trials of drugs that are not available in the UK and were compared with placebo only (that is, would not contribute to estimates of other interventions).
The evidence reviewed in this chapter is organised into five major sections: (1) pharmacological interventions (see Section 6.6), (2) psychological interventions (see Section 6.7), (3) combination interventions (see Section 6.8), (4) specific subgroups (see Section 6.9) and (5) health economic evidence (see Section 6.10). The clinical summary, evidence to recommendations and clinical recommendations appear at the end of the chapter. The chapter includes results from the NMA and from pairwise analyses (see Table 11, Table 12, Table 13, Figure 5 and Figure 6.
The GDG had concerns about the comparability of participants in different trials. In particular, participants in pharmacological and psychological trials may differ insofar as users find different interventions more or less tolerable in light of their personal circumstances and preferences. Similarly, self-help trials may recruit participants who would not seek or accept face-to-face interventions. However, large trials have successfully recruited participants who are willing to be randomised to either medication or psychotherapy and to either self-help or face-to-face treatment. Moreover, some participants in psychological intervention trials (typically 25%) were already taking antidepressants and other medication. The NMA assumes that users are willing to accept any of the interventions included; in practice, treatment decisions will be restricted by individual values and goals.
The different results are distinguished by different labels: results labelled ‘SMDN’ are taken from the NMA and those labelled ‘SMD’ are from a pairwise analysis. For all analyses, the number of participants reported is the number receiving treatment who were included in the analysis. For both the NMA and pairwise analyses, the GDG was first interested in the effects for major classes of interventions (for example, SSRIs and individual CBT) and secondly in any differences among members of those classes (for example, between specific drugs). The NMA includes effects for each class and for each member of the class (see Chapter 3 for the method). Pairwise analyses include overall effects for each class, each subgroup and tests for differences among subgroups (for example, different drugs or variations of a therapy). Within each major section, results are organised alphabetically by class and alphabetically by intervention within the class.
In estimating symptoms of social anxiety, all effects are taken from the NMA unless otherwise specified. The structure of the NMA is included in Appendix 11. Effect sizes from the NMA are presented relative to waitlist. As described (see Chapter 3), the relative effects of any two interventions in the NMA can be calculated by subtraction (that is, the choice of baseline comparator for reporting does not affect the results). In addition to estimating active treatments, effects were estimated for pill placebo and for psychological placebo. Results are reported as mean values with 95% credible intervals (CrI), which are analogous to confidence intervals in frequentist statistics (see Table 10).
Table 10
Effects for control groups in the network meta-analysis.
Further details about the review are included in the appendices. The complete search strategy and PRISMA17 chart can be found in Appendix 6. Forest plots for pairwise analyses are included in Appendix 14, and GRADE profiles for pairwise analyses are included in Appendix 15. Study characteristics are included in Appendices 12 and 14.
6.5.1. Network meta-analysis of social anxiety disorder post-treatment
Trials included in the NMA included between 18 and 839 participants at baseline (median 78). Where known, participants were on average (median of means) 36 years old and 80% white. About half of the included participants were female (52%). There were no participants on medication in 44 trials, including most of the pharmacological trials, and it was unclear in 27 trials if participants were taking medication at baseline. In the remaining 27 trials, approximately 27% of participants were taking medication at the start (see Appendix 12).
Quality of the evidence
To rate the quality of evidence, guidelines may use GRADE profiles for critical outcomes. However, GRADE has not yet been adapted for use in NMAs. To evaluate the quality of the evidence from the NMA, information about the factors that would normally be included in a GRADE profile (that is, risk of bias, publication bias, imprecision, inconsistency and indirectness) are reported. Additionally, before conducting the NMA, the results of pairwise comparisons were presented to the GDG and the quality of the included trials and the evidence for each outcome and comparison were discussed. Study quality and risk of bias (see below) were assessed for all studies, irrespective of whether they were included in the NMA or pairwise comparisons.
Risk of bias
All included trials were assessed for risk of bias (see Appendix 20 and Figure 5). Of those in the NMA, 74 were at low risk for sequence generation and 69 of these were at low risk of bias for allocation concealment. Trials of psychological interventions were considered at high risk of bias for participant and provider blinding per se, and the rate of side effects may also make it difficult to maintain blinding in pharmacological trials. Most reported outcomes were self-rated, but assessor blinding was considered separately for all trials; 94 were at low risk of bias (no assessor-rated outcomes or assessors blind), one was unclear and assessors were aware of treatment conditions in five trials. For incomplete outcome data, 71 trials were at low risk of bias; it was unclear how missing data were handled in four trials and 25 were at high risk of bias (for example, those that reported per protocol or completer analyses and those with very high amounts of missing data).

Figure 5
Risk of bias summary.
Selective outcome reporting and publication bias
Several methods were employed to minimise risk of selective outcome reporting and publication bias. All authors were contacted in writing to request trial registrations and unpublished outcomes, and all authors of included trials, stakeholders and pharmaceutical manufacturers were asked to provide unpublished trials. Nonetheless, most of the included trials were not registered. Only 30 were at low risk of selective outcome reporting bias; 53 were at unclear risk of bias and 18 were at high risk of bias. Trials of psychological and pharmacological interventions were equally likely to be at unclear risk of bias. For interventions developed before the introduction of mandatory trial registration, results may be particularly overestimated as a result of publication bias.
Inconsistency
The random effects model was a good fit with the data, although the between-trials SD (heterogeneity) had a posterior median of 0.19 with 95% credible interval (0.14, 0.25).
Inconsistency was assessed by fitting an unrelated mean effects model (Dias et al., 2012) and comparing the fit with that of the full NMA model using the residual deviance (Dias et al., 2012). There was no evidence of inconsistency in the NMA. The posterior mean of the residual deviance for the NMA model was 164.0 compared with 169.3 in the independent effect mode (lower values are favoured). The results of the NMA were also consistent in magnitude and direction with the results of pairwise comparisons.
Indirectness
All evidence in the NMA is direct insofar as it relates to the population and outcomes of interest. The sections that follow describe the direct comparisons that have been made among the interventions included in the NMA.
The GDG had concerns about the comparability of participants in different trials. In particular, participants in pharmacological and psychological trials may differ insofar as users find different interventions more or less tolerable in light of their personal circumstances and preferences. Similarly, self-help trials may recruit participants who would not seek or accept face-to-face interventions. However, large trials have successfully recruited participants who are willing to be randomised to either medication or psychotherapy and to either self-help or face-to-face treatment. Moreover, some participants in psychological intervention trials (typically 25%) were already taking antidepressants and other medication. The NMA assumes that users are willing to accept any of the interventions included; in practice, treatment decisions will be restricted by individual values and goals.
Table 11
Results from the NMA – summary of treatment and class effects compared with waitlist.
Table 12
Results of pairwise comparisons –symptoms of social anxiety at post-treatment.
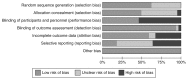
Figure 6
Results of pairwise comparisons – risk of bias summary chart.
6.6. PHARMACOLOGICAL INTERVENTIONS
6.6.1. Anticonvulsants
Five trials (FELTNER2011, PANDE1999, PANDE2004, PFIZER2007, ZHANG2005) that evaluated anticonvulsants (gabapentin, levetiracetam and pregabalin) were included in the NMA (242 participants on treatment). Effects for each drug were similar to the medium average effect for the class (SMDN = −0.81, 95% CrI = −1.36 to −0.28). All anticonvulsants were significantly different from waitlist.
Gabapentin
One trial (PANDE1999) compared gabapentin (34 participants on treatment) with placebo. While the mean dose at endpoint was not reported, 56% of participants reached the maximum dose of 3,600 mg per day by the end of the 14-week trial. At post-treatment there was a large effect compared with waitlist (SMDN = −0.89, 95% CrI = −1.41 to −0.37).
Levetiracetam
One trial (ZHANG2005) compared levetiracetam (nine participants on treatment) with placebo. Participants received a mean dose of 1,140 mg twice a day for 7 weeks. At post-treatment, there was a medium effect compared with waitlist (SMDN = −0.83, 95% CrI = −1.48 to −0.18).
Pregabalin
Three trials (FELTNER2011, PANDE2004, PFIZER2007) compared pregabalin (199 participants on treatment) with placebo. Participants in two trials received a fixed daily dose of 600 mg; participants in the other trial received a fixed daily dose of 400 mg. Trials lasted 10 or 11 weeks. At post-treatment, there was a medium effect compared with waitlist (SMDN = −0.72, 95% CrI = −1.07 to −0.37).
In two trials (PANDE2004, PFIZER2007), fixed doses at the starting level of the BNF recommended prescription range were excluded from the NMA (150 and 200 mg per day) as the GDG considered these unlikely to be clinically effective and unrepresentative of practice.
6.6.2. Benzodiazepines
Five trials (DAVIDSON1993, GELERNTER1991, KNIJNIK2008, MUNJACK1990, OTTO2000) that evaluated the benzodiazepines alprazolam and clonazepam were included in the NMA (112 participants on treatment). Effects for each drug were similar to the large average effect for the class (SMDN = −0.96, 95% CrI = −1.56 to −0.35) and they were significantly different from waitlist.
Alprazolam
One trial (GELERNTER1991) compared alprazolam (12 participants on treatment) with placebo, phenelzine or CBT. Participants received a mean end dose of 4.2 mg per day for 12 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.85, 95% CrI = −1.40 to −0.29).
Clonazepam
Four trials (DAVIDSON1993, KNIJNIK2008, MUNJACK1990, OTTO2000) included a group that received clonazepam (100 participants on treatment), compared with placebo, waitlist, psychodynamic psychotherapy plus clonazepam, or group CBT. Participants received 2.4 to 4.0 mg of clonazepam daily for 8 to 12 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −1.07, 95% CrI = −1.44 to −0.70).
6.6.3. Monoamine oxidase inhibitors
Ten trials (BLANCO2010, BURROWS1997, GELERNTER1991, HEIMBERG1998, LIEBOWITZ1990, OOSTERBAAN2001, PRASKO2003, SCHNEIER1998, STEIN2002a, VERSIANI1992) evaluating MAOIs were included in the NMA (615 participants on treatment); the large effect on symptoms of social anxiety for the class (SMDN = −1.01, 95% CrI = −1.56 to −0.45) was between effects for moclobemide and phenelzine. Both interventions were significantly different from waitlist. One MAOI (brofaromine) was not included in the NMA because it is no longer manufactured, but the GDG considered it might have similar effects and side effects to those that are currently available; it was included in a sensitivity analysis.
In a pairwise analysis of two trials (GELERNTER1991, OOSTERBAAN2001; 37 participants on treatment), there was no evidence of an effect on symptoms of anxiety at follow-up compared with placebo (SMD = −0.27, 95% CI = −1.05 to 0.51) with substantial heterogeneity (I2 = 67%; Chi2 = 9.09, p = 0.03).
In seven trials (BLANCO2010, BURROWS1997, HEIMBERG1998, LIEBOWITZ1990, OOSTERBAAN2001, SCHNEIER1998, VERSIANI1992; 393 participants on treatment), there was a small effect on depression at post-treatment (SMD = −0.22, 95% CI = −0.37 to −0.07) with considerable heterogeneity (I 2 = 77%; Chi2 = 30.02, p = 0.0001). One trial (17 participants on treatment) reported no evidence of an effect on depression at follow-up (SMD = 0.30, 95% CI = −0.39 to 0.99). In the same trials (383 participants on treatment), there was a moderate effect on disability at post-treatment (SMD = −0.54, 95% CI = −0.95 to −0.12) with considerable heterogeneity (I 2 = 82%; Chi2 = 39.44, p = <0.00001). In two trials (GELERNTER1991, OOSTERBAAN2001; 29 participants on treatment) there was no evidence of an effect on disability at follow-up (SMD = −0.11, 95% CI = −0.66 to 0.43) with no significant heterogeneity (I 2 = 18%; Chi2 = 1.22, p = 0.27). No trials reported a measure of quality of life.
In two trials (NOYES1997 [Noyes et al., 1997], SCHNEIER1998; 123 participants on treatment), the effect was not statistically significant for withdrawal because of side effects compared with placebo (RR = 1.13, 95% CI = 0.63 to 2.05) with no significant heterogeneity (I 2 = 0%; Chi2 = 0.55, p = 0.46). There was also no evidence of an effect on the total number of people experiencing any adverse event (RR = 1.09, 95% CI = 0.97 to 1.23; 381 participants on treatment) with no heterogeneity.
Moclobemide
Six trials (BURROWS1997, OOSTERBAAN2001, PRASKO2003, SCHNEIER1998, STEIN2002a, VERSIANI1992) included one or more groups who received moclobemide (490 participants on treatment); six were included in the NMA. Participants received 581 to 728 mg daily for 8 to 26 weeks. All included trials included a placebo comparison and one also compared moclobemide with phenelzine. At post-treatment, there was a medium effect compared with waitlist (SMDN = −0.73, 95% CrI = −1.03 to −0.44).
One group in an included trial (BURROWS1997) was below the recommended range in the BNF prescription range and was excluded from the review (300 mg per day).
Other trials were not included in the NMA either because the authors did not report data that could be included in meta-analysis (NOYES1997) or because they included a study population with very severe symptoms (ATMACA2002 [Atmaca et al., 2002]). While many trials included only participants scoring above 70 on the LSAS and had mean values close to the cut-off, participants in ATMACA2002 (36 participants on treatment) scored 122 at baseline; there was a small effect (favouring citalopram) on symptoms of social anxiety at post-treatment (SMD = −0.36, 95% CI = −0.69 to −0.03) and there was no evidence of an effect between the groups on the number of people reporting any adverse event (RR = 1.19, 95% CI = 0.56 to 2.51). No controlled follow-up data were reported.
Phenelzine
Five trials (BLANCO2010, GELERNTER1991, HEIMBERG1998, LIEBOWITZ1990, VERSIANI1992) included one or more groups receiving phenelzine (125 participants on treatment) and were included in the NMA. All included a placebo comparison and one also compared phenelzine with moclobemide, as noted above. Participants received 55 to 76 mg daily for 8 to 12 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −1.28, 95% CrI = −1.57 to −0.98).
Tranylcypromine
One trial compared tranylcypromine in fixed daily doses of 30 mg and 60 mg for 12 weeks; it could not be included in the NMA because there was neither a placebo group nor another intervention that was included in the network (NARDI2010; 17 participants on treatment). There was large effect on symptoms of social anxiety disorder at post-treatment favouring the higher dose (SMD = −0.85, 95% CI = −1.54 to −0.17) and the effect was not statistically significant for dose on the number per group reporting at least one adverse event (RR = 0.84, 95% CI = 0.61 to 1.15).
Brofaromine (sensitivity analysis)
Three trials compared brofaromine with placebo (FAHLEN1995, LOTT1997, VAN-VLIET1992) and were not included in the NMA because brofaromine is no longer manufactured. A pairwise analysis was conducted comparing brofaromine (101 participants on treatment) with placebo. Participants received 107 to 150 mg daily for 12 weeks. There was a medium effect compared with placebo at post-treatment (SMD = −0.71; 95% CI = −1.08 to −0.34) with no important heterogeneity (I2 = 36%; Chi2 = 3.12%, p = 0.0002). There was no difference in the overall effect of MAOIs versus placebo either with (SMD = −0.58; 95% CI = −0.81 to −0.34) or without (SMD = −0.53; 95% CI = −0.81 to −0.25) the brofaromine trials (see Appendix 14 for the forest plots). One trial reported controlled results at follow-up, but only one participant remained in the placebo group and the data were not analysed.
6.6.4. Selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs)
Twenty-five trials (ALLGULANDER1999, ALLGULANDER2004, ASAKURA2007, BALDWIN1999, BLOMHOFF2001, DAVIDSON2004a, FURMARK2002, FURMARK2005, GSK2006, KASPER2005, LADER2004, LEPOLA2004, LIEBOWITZ2002, LIEBOWITZ2003, LIEBOWITZ2005a, LIEBOWITZ2005b, PFIZER2007, RICKELS2004, SEEDAT2004, STEIN1998, STEIN1999, STEIN2005, VAN-AMERINGEN2001, VAN-VLIET1994, WESTENBERG2004) evaluating SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline) and SNRIs (venlafaxine) were included in the NMA (4,043 participants on treatment). At post-treatment, effects for each drug were similar to the average effect for the class compared with waitlist (SMDN = −0.91, 95% CrI = −1.22 to −0.60) and SSRIs/SNRIs were significantly different from waitlist.
SSRIs
In a pairwise analysis of two trials (ALLGULANDER1999, BLOMHOFF2001; 210 participants on treatment), there was no evidence of an effect on symptoms of anxiety at follow-up compared with placebo (SMD = −0.08, 95% CI = −0.32 to 0.16) with moderate heterogeneity between trials (I2 = 32%; Chi2 = 2.95, p = 0.23).
One trial (BLOMHOFF2001) reported a medium effect on quality of life at post-treatment (193 participants on treatment) (SMD = −0.41, 95% CI = −0.82 to 0.00) and no evidence of an effect at follow-up (168 participants on treatment (SMD = − 0.24, 95% CI = −0.71 to 0.24). In 11 trials (BALDWIN1999, CLARK2003, DAVIDSON2004a, DAVIDSON2004b, GSK2006, KOBAK2002, LADER2004, LEPOLA2004, LIEBOWITZ2003, PFIZER2007, VAN-VLIET1994; 1,736 participants on treatment), there was a small effect on depression at post-treatment (SMD = −0.20, 95% CI = −0.29 to −0.12) with no significant heterogeneity (I 2 = 8%; Chi2 = 15.17, p = 0.37). In 14 trials (ALLGULANDER1999, ASAKURA2007, BLOMHOFF2001, DAVIDSON2004a, FURMARK2005, KOBAK2002, LADER2004, LEPOLA2004, LIEBOWITZ2003, PFIZER2007, STEIN1998, STEIN1999, VAN-VLIET1994, WESTENBERG2004; 1,987 participants on treatment), there was a medium effect on anxiety-related disability at post-treatment (SMD = −0.57, 95% CI = −0.71 to −0.42) with considerable heterogeneity between trials (I2 = 71%; Chi2 = 59.54, p < 0.00001) and between subgroups (I2 = 68.8%; Chi2 = 16.04, p = 0.007). In two trials (ALLGULANDER1999, BLOMHOFF2001; 210 participants on treatment), there was a small effect on anxiety-related disability at follow-up (SMD = −0.24, 95% CI = −0.52 to −0.04) with no significant heterogeneity between trials (I2 = 49%; Chi2 = 3.91, p = 0.14).
In 17 trials (ALLGULANDER1999, ALLGULANDER2004, ASAKURA2007, BALDWIN1999, CLARK2003, DAVIDSON2004a, FURMARK2005, GSK2006, KASPER2005, LADER2004, LEPOLA2004, LIEBOWITZ2005B, PFIZER2007, STEIN1998, STEIN1999, VAN-AMERINGEN2001, VAN-VLIET1994; 2,488 participants on treatment), there was a large effect on withdrawal because of side effects compared with placebo at post-treatment (RR = 2.35, 95% CI = 1.80 to 3.08) with no significant heterogeneity (I2 = 0%; Chi2 = 18.28, p = 0.50). Differences between subgroups were not significant (I2 = 25.6%; Chi2 = 5.38, p = 0.25). In 11 trials (ALLGULANDER2004, ASAKURA2007, BALDWIN1999, DAVIDSON2004a, GSK2006, LADER2004, LIEBOWITZ2005b, PFIZER2007, STEIN1999, VAN-VLIET1994, WESTENBERG2004; 1,978 participants on treatment), there was a small effect on the number of participants reporting any adverse event (RR = 1.18, 95% CI = 1.11 to 1.25) with substantial heterogeneity between individual trials (I2 = 56%; Chi2 = 32.03, p = 0.004) but not between subgroups (I2 = 0%; Chi2 = 0.04, p = 0.98).
Citalopram
Two trials (FURMARK2002, FURMARK2005) included a group receiving citalopram (18 participants on treatment) compared with placebo and were included in the NMA. Participants received 40 mg daily for 6 and 9 weeks. At post-treatment, there was a medium effect compared with waitlist (SMDN = −0.83, 95% CrI = −1.27 to −0.39).
Escitalopram
Two trials (KASPER2005, LADER2004) included one or more groups receiving escitalopram (675 participants on treatment) compared with placebo. Participants received 5 to 20 mg daily for 12 and 24 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.88, 95% CrI = −1.19 to −0.56).
Fluoxetine
Three trials (CLARK2003, DAVIDSON2004b, KOBAK2002) included a group receiving fluoxetine (107 participants on treatment) compared with placebo, individual CT or group CBT. In one trial (CLARK2003), participants receiving fluoxetine and placebo were instructed to expose themselves to feared situations. Participants received a mean dose of between 44 and 60 mg daily for 12 and 24 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.87, 95% CrI = −1.16 to −0.57).
Fluvoxamine
Five trials (ASAKURA2007, DAVIDSON2004a, STEIN1999, VAN-VLIET1994, WESTENBERG2004) included participants receiving fluoxetine (500 participants on treatment) compared with placebo. Participants received 150 to 225 mg daily for 12 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.94, 95% CrI = −1.25 to −0.63).
Paroxetine
Eleven trials (ALLGULANDER1999, ALLGULANDER2004, BALDWIN1999, GSK2006, LADER2004, LEPOLA2004, LIEBOWITZ2002, LIEBOWITZ2005b, PFIZER2007, SEEDAT2004, STEIN1998) included one or more groups receiving paroxetine (1,449 participants on treatment) compared with placebo, escitalopram or venlafaxine. Participants received a mean dose of between 20 and 46 mg daily. Ten trials lasted between 10 and 12 weeks; one lasted 24 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.99, 95% CrI = −1.26 to −0.73).
One group in an included trial (LIEBOWITZ2002) was outside the recommended BNF prescription range and was excluded from the review (60 mg per day).
Sertraline
Three trials (BLOMHOFF2001, LIEBOWITZ2003, VAN-AMERINGEN2001) included one or more groups receiving sertraline (535 participants on treatment) compared with placebo. Participants received 120 to 159 mg daily for 12 to 24 weeks. Two groups of participants receiving sertraline and placebo were instructed to expose themselves to feared situations in BLOMHOFF2001. At post-treatment, there was a medium effect compared with waitlist (SMDN = −0.91, 95% CrI = −1.22 to −0.61).
SNRIs
Venlafaxine
In five trials (ALLGULANDER2004, LIEBOWITZ2005a, LIEBOWITZ2005b, RICKELS2004, STEIN2005; 759 participants on treatment) comparing venlafaxine with placebo, a higher dose of venlafaxine or paroxetine, participants received 72 to 213 mg daily for 12 to 28 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.96, 95% CrI = −1.25 to −0.67).
In three trials (ALLGULANDER2004, LIEBOWITZ2005b, STEIN2005; 542 participants on treatment), there was a large effect of venlafaxine withdrawal due to side effects at post-treatment (RR = 2.51, 95% CI = 1.57 to 4.02) with no heterogeneity. In three trials (ALLGULANDER2004, LIEBOWITZ2005a, RICKELS2004; 411 participants on treatment), there was a small effect on the number of people reporting any adverse event (RR = 1.10, 95% CI = 1.04 to 1.15) with no heterogeneity. None of the trials reported measures of quality of life, depression or anxiety-related disability.
Duloxetine
One trial (SIMON2010) comparing duloxetine in fixed daily doses of 60 mg and 120 mg for 18 weeks (15 participants on treatment) following a 6-week open-label study of 60 mg of duloxetine could not be included in the NMA because there was neither a placebo group nor another intervention that was included in the network. There was a large effect on symptoms of social anxiety at post-treatment favouring the higher dose (SMD = −1.22, 95% CI = 0.39 to 2.05).
6.6.5. Other pharmacological interventions
Mirtazapine (noradrenaline and selective serotonin antagonist)
Two trials compared mirtazapine with placebo. One was excluded from the NMA because the reported data included improbable figures that the journal and the authors were unable to verify (MUEHLBACHER2005 [Muehlbacher et al., 2005]) despite contacting the study authors directly. In one included trial (SCHUTTERS2010) comparing mirtazapine with placebo, participants (30 participants on treatment) received 40 mg daily for 12 weeks (SCHUTTERS2010). At post-treatment, there was a medium effect compared with waitlist (SMDN = −0.80, 95% CrI = −1.45 to −0.16).
Antipsychotics
The GDG decided a priori not to include trials of antipsychotics in the NMA because they are not used in the primary treatment of social anxiety disorder and the GDG was also concerned that participants in these trials would likely differ from the participants in other trials.
One trial (VAISHNAVI2007) compared quetiapine (ten participants on treatment) with placebo. Participants received 147 mg daily for 8 weeks. There was no evidence of an effect on symptoms of social anxiety disorder at post-treatment (SMD = −0.28; 95% CI = −1.36 to 0.81). In addition to the negative result from this trial, searches identified several completed but unpublished trials of quetiapine for social anxiety disorder.
One trial (BARNETT2002) compared olanzapine (four participants on treatment) with placebo. Participants received a mean daily dose of 9 mg for 8 weeks. There was a large effect on symptoms of social anxiety at post-treatment (SMD = −2.28, 95% CI = −4.00 to −0.55). No controlled follow-up data were reported.
Several completed trials have never been reported and are not included here.
Paroxetine combined with clonazepam
In one trial (SEEDAT2004) comparing combination treatment of paroxetine and clonazepam (14 participants on treatment) with paroxetine alone, participants received combined treatment for 10 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −1.35, 95% CrI = −1.93 to −0.78).
6.6.6. Continued pharmacotherapy for relapse prevention
Selective serotonin reuptake inhibitors
In four trials (KUMAR1999, MONTGOMERY2005, STEIN2002b, VAN-AMERINGEN2001), participants who met criteria for response to a SSRI (paroxetine, escitalopram, or sertraline) were randomly assigned to receive continued treatment (365 participants on treatment) or placebo (see Appendix 18 for the study characteristics). Continued pharmacotherapy was associated with lower relapse (RR = 0.47, 95% CI = 0.27 to 0.82), with approximately 23% of participants on treatment and 54% of participants receiving placebo (unweighted means) relapsing by 16 to 24 weeks after the start of the relapse prevention study (see Table 13 and Appendix 26 for the forest plots and Appendix 15 for the GRADE profiles).
Table 13
Results of pairwise comparisons – relapse prevention.
Anticonvulsants
One trial (GREIST2011) randomised participants meeting criteria for response in a 10-week open-label study of pregabalin to receive pregabalin (80 participants on treatment) or placebo (see Appendix 18 for the study characteristics). After 26 weeks of double-blind treatment, the effect was not statistically significant for relapse (RR = 0.79, 95% CI = 0.58 to 1.06) and the majority of people for whom outcomes were known had relapsed in both groups (63% having treatment; 71% taking placebo) (see Table 13 and Appendix 26 for the forest plots and Appendix 15 for the GRADE profiles).
6.6.7. Additional considerations concerning the use of medication for social anxiety disorder
The GDG was aware of the limited evidence available in the trials of the tolerability, side effects and other potential harms (for example, interactions with other prescribed medication) associated with the use of the drugs reviewed. The GDG therefore considered whether additional sources of information could be identified that could inform the development of recommendations for the use of medication in the treatment of people with social anxiety disorder.
The GDG decided, based on an application of the rules for extrapolation and adaptation/incorporation (see Chapter 3), that the guidance developed for the use of the drugs in other anxiety disorders and depression (Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults [NICE, 2011c] and Depression [NICE, 2009a]) could be relevant to social anxiety disorder. Specifically, the GDG considered that there were sufficient commonalities to justify the extrapolation, namely: (a) underlying aetiologies and aspects of presentation in depression and other anxiety disorders and in social anxiety disorder; (b) a high comorbidity between the disorders; and (c) similar modes of action for both the therapeutic and non-therapeutic aspects of drug use. The previous guidelines were considered sufficiently recent for the purpose of this current guideline. Although the previous guidelines considered effects on symptoms of relevant mental disorders, for the purpose of this guideline, the GDG considered only evidence of side effects, which should not differ in people with social anxiety disorder, generalised anxiety disorder or depression (for example, effects on physical health). In addition the GDG also considered those aspects of the presentation of depression and other anxiety disorders and social anxiety disorder that can differ (for example, the impact on social interaction) in reviewing the evidence in Depression. Finally, the GDG reviewed the recommendations concerning the safety and tolerability of relevant drugs in Generalised Anxiety Disorder and Panic Disorder. A topic group undertook the initial reviews described in this section and presented a summary to the GDG. The GDG used this summary and their own knowledge and expertise to develop the recommendations using an informal consensus method.
Reviews of existing NICE guidelines
The key elements of the reviews of side effects and other harms of medication in Generalised Anxiety Disorder and Panic Disorder (NICE, 2011c) and Depression (NICE, 2009a) that were identified by the GDG as being relevant to this current guideline are summarised below.
Cardiovascular
Anxiety disorders, including social anxiety disorder, are associated with an increased mortality risk (Mykletun et al., 2009). TCAs are associated with higher risk of developing cardiovascular adverse events and have found to be cardiotoxic in overdose (Taylor, 2008). In contrast to the concerns about the TCAs relatively little concern has been raised about the potential cardiotoxicity of the SSRIs, although a recent warning about the QTc prolongation and the use of citalopram was raised by the Medicines and Healthcare products Regulatory Agency (MHRA, 2011). Indeed, SSRIs do not appear to be associated with an increase risk in cardiovascular adverse events (for example, Swenson and colleagues [2006], and Taylor [2008]) and are associated with a relatively low fatal toxicity index (number of poisoning deaths per 1,000,000 prescriptions).
Other non-SSRI drugs considered by the GDG in the evidence review, including mirtazapine and moclobemide, were also not identified by Depression (NICE, 2009a) as conferring particular risk in overdose. In contrast, phenelzine can cause postural hypotension particularly in the early weeks of treatment and may also be associated with significant bradycardia. However, its fatal toxicity index in overdose appears to be less than most TCAs. Concern has been raised about venlafaxine with some evidence of increased blood pressure in higher doses and concern about a higher fatal toxicity index in overdose than SSRIs (Buckley & McManus, 2002; Taylor, 2008). Duloxetine has been associated with small increases in diastolic blood pressure, tachycardia and cholesterol compared with placebo (Dugan & Fuller, 2004; Wernicke et al., 2007).
Bleeding
Observational studies using data from national prescribing databases have found a relatively strong association (approximately three-fold increase in risk of bleeding) between SSRIs and increased risk of gastrointestinal bleeding (Weinrieb et al., 2003; Yuan et al., 2006). However, it should be noted that the outcome was a rare event, with approximately four to five events per 1000 person years. This effect was stronger (approximately 15-fold increase of bleeding) in people concurrently using non-steroidal anti-inflammatory drugs and SSRIs and the risk may be increased in older people.
Gastrointestinal symptoms
There is evidence both in depression and anxiety disorders of an increased risk of gastrointestinal symptoms such as nausea, vomiting and diarrhoea associated with SSRI use (Beasley et al., 2000; Brambilla et al., 2005). TCAs may be associated with higher risk of constipation when compared with fluoxetine (Beasley et al., 2000). This was supported by the review undertaken by in Generalised Anxiety Disorder and Panic Disorder (NICE, 2011c).
Sexual dysfunction
There was consistent evidence of sexual adverse effects in association with SSRIs, duloxetine and venlafaxine in people with depression (Beasley et al., 2000; Gregorian et al., 2002; Keller, 2000; Werneke et al., 2006).
Weight
Fluoxetine appears to be associated with greater weight loss compared with placebo (Beasley et al., 2000), TCAs and other SSRIs (Brambilla et al., 2005). However, it was noted in Generalised Anxiety Disorder and Panic Disorder (NICE, 2011c) that there is a possibility that paroxetine and fluoxetine may actually be associated with weight gain, but this needs further research to establish this finding. There is some evidence that duloxetine was associated with weight loss with a mean reduction of 2.2 kg compared with 1 kg for placebo (Dugan & Fuller, 2004). Pregabalin is associated with weight increase (Cabrera et al., 2012).
Cognitive/neurological
Pregabalin was reported in Generalised Anxiety Disorder and Panic Disorder to be reasonably well tolerated but could for some people give rise to headaches, dizziness and somnolence. In contrast, benzodiazepines were associated with a number of cognitive side effects including impairment in speech and memory along with sedation, fatigue and ataxia. However, the most commonly reported problem with benzodiazepine use was risk of dependence. This suggests only short-term use of this treatment is appropriate and that particular caution should be exercised for people with comorbid alcohol or drug misuse.
Discontinuation
A specific issue that the GDG considered important, and which supported extrapolation from the evidence reviews in Depression, included a focus on ‘discontinuation’ rather than ‘withdrawal’ symptoms because the GDG for the current guideline accepted the view set out in Depression that drugs commonly used in the treatment of depression (for example, the SSRIs) are not addictive. However, the GDG did accept the view (as with depression) that some discontinuation symptoms may be hard to distinguish from the underlying symptoms of social anxiety disorder. Following Depression, the GDG divided discontinuation symptoms into six groups, which by definition are not attributable to other causes: (1) affective (for example, irritability); (2) gastrointestinal (for example, nausea); (3) neuromotor (for example, ataxia); (4) vasomotor (for example, sweating); (5) neurosensory (for example, paraesthesia); and (6) other neurological (for example, dreaming) (Delgado, 2006). They are experienced by at least a third of patients taking SSRIs (Lejoyeux et al., 1996; MHRA, 2004) and are seen to some extent with all antidepressants (Taylor et al., 2006). Of the commonly used antidepressants, the risk of discontinuation symptoms seems to be greatest with paroxetine, venlafaxine and amitriptyline (Taylor et al., 2006). Depression considered a number of prospective studies examining the effect of discontinuation in people taking a range of antidepressants. These studies suggest an increase in discontinuation symptoms in those taking paroxetine compared with escitalopram (Baldwin et al., 2006), fluoxetine (Bogetto et al., 2002; Hindmarch et al., 2000; Judge et al., 2002; Michelson et al., 2000; Rosenbaum et al., 1998), sertraline (Hindmarch et al., 2000; Michelson et al., 2000), citalopram (Hindmarch et al., 2000) and venlafaxine when compared with escitalopram (Montgomery et al., 2004) or sertraline (Sir et al., 2005).
The onset of discontinuation symptoms is usually within 5 days of stopping treatment, or occasionally during taper or after missed doses (Michelson et al., 2000; Rosenbaum et al., 1998). This is influenced by a number of factors, which may include a drug's half-life. Symptoms can vary in form and intensity and occur in any combination. They are usually mild and self-limiting, but can be severe and prolonged, particularly if withdrawal is abrupt. Some symptoms are more likely with individual drugs, for example, dizziness and electric shock-like sensations with SSRIs, and sweating and headache with TCAs (Haddad, 2001; Lejoyeux et al., 1996). Although anyone can experience discontinuation symptoms, the risk is increased in those prescribed short half-life drugs (Rosenbaum et al., 1998), such as paroxetine and venlafaxine (Fava et al., 1997; Hindmarch et al., 2000; MHRA, 2004). They can also occur in people who do not take their medication regularly. Two-thirds of people prescribed SSRIs and other related drugs skip a few doses from time to time (Meijer et al., 2001). The risk is also increased in the following people: those who have been taking the drugs for 8 weeks or longer (Haddad, 2001); those who developed anxiety symptoms at the start of antidepressant treatment (particularly with SSRIs); those receiving other centrally acting medications (for example, antihypertensives, antihistamines, antipsychotics); children and young people; and those who have experienced discontinuation symptoms before (Haddad, 2001; Lejoyeux & Ades, 1997).
Although it is generally advised that antidepressants (except fluoxetine) should be discontinued over a period of at least 4 weeks, preliminary data suggest that it may be the half-life of the antidepressant rather than the rate of taper that ultimately influences the risk of discontinuation symptoms (Tint et al., 2008). When switching from one antidepressant to another with a similar pharmacological profile, the risk of discontinuation symptoms may be reduced by completing the switch as quickly as possible (within a few days at most). A different approach may be required at the end of treatment where a slower taper is likely to be beneficial. People taking MAOIs may need the dosage to be tapered over a longer period (Haddad, 2001). Tranylcypromine may be particularly difficult to stop. It is not clear if the need for slow discontinuation of MAOIs, and particularly tranylcypromine, is due to the discontinuation syndrome or the loss of other neurochemical effects of these drugs.
Many people experience discontinuation symptoms despite a slow taper. For these, the option of abrupt withdrawal should be discussed. Some may prefer a short period of intense symptoms rather than a prolonged period of milder symptoms. There are no systematic randomised studies in this area, therefore treatment is pragmatic. Mild symptoms are not uncommon after discontinuing an antidepressant and they will pass in a few days. For severe symptoms the original antidepressant (or another with a longer half-life from the same class) can be reintroduced and tapered gradually while monitoring for symptoms (Haddad, 2001; Lejoyeux & Ades, 1997).
Suicidal ideation and suicidal behaviour
The Depression guideline was particularly concerned with suicide because depression is the largest cause of suicide, with two-thirds of people who attempt suicide experiencing depression, and suicide is the main cause of the increased mortality of depression. Suicidal ideation may also be present in anxiety disorders, particularly if comorbid with depression (Nepon et al., 2010). In a systematic review, Stone and colleagues (2009) identified the association between antidepressant use and suicidal ideation and/or suicidal behaviour. For those under 25 years, there were increased odds of suicidal behaviour (odds ratio 2.30; 95% CI = 1.04, 5.09) associated with antidepressants compared with placebo. There was a borderline statistically significant increase in the odds of suicidal ideation and suicidal behaviour (odds ratio 1.62; 95% CI = 0.97, 2.71). Two meta-analyses of RCTs (Fergusson et al., 2005; Gunnell et al., 2005) (k = 702 and k = 477, respectively) and a large nested case-control study comparing new prescriptions of SSRIs and TCAs (Martinez et al., 2005) found no evidence of an increase in completed suicide with SSRIs but possible evidence of increased suicidal/self-harming behaviour with SSRIs compared with placebo (the number needed to harm was 684 and 754 in the two meta-analyses). There was no overall difference between SSRIs and TCAs, but there was some evidence for increased self-harming behaviour with SSRIs compared with TCAs in people under 19 years (Fergusson et al., 2005; Martinez et al., 2005). In a similar vein, a review by Möller and colleagues (2008) concluded that all antidepressants carry a small risk of inducing suicidal thoughts and suicide attempts in those aged below 25 years with the risk reducing at about 30 to 40 years of age.
There may be a delay in noticeable improvement after starting antidepressants, and, just after initiation of treatment, mood remains low with prominent feelings of guilt and hopelessness, but energy and motivation can increase and it has been hypothesised that this may be related to increased suicidal thoughts. A similar situation can arise with people who develop akathisia or increased anxiety due to a direct effect of some SSRIs and related drugs, which may increase the propensity to suicidal ideation and suicidal behaviour (Healey, 2003). Careful monitoring was therefore recommended by the Depression guideline when treatment is initiated with an antidepressant. The guideline also recommended that people taking antidepressants should be monitored regardless of the apparent severity of their depression.
A meta-analysis of observational studies (Barbui et al., 2009) found that compared with people with depression who did not take antidepressants, young people taking SSRIs had a significantly higher risk of suicide attempts and completed suicide. In contrast, adults (especially older adults) had a significantly lower risk of suicide attempts and completed suicide.
Risk in overdose
Antidepressants can be toxic in overdose and given elevated levels of suicidality with some anxiety disorders the use of antidepressants is of concern. Antidepressants were involved in 18% of deaths from drug poisoning between 1993 and 2002 (Morgan et al., 2004), with TCAs, which are cardiotoxic in overdose, accounting for 89% of these. This is equivalent to 30.1 deaths per 1,000,000 prescriptions. Dothiepin/dosulepin alone accounted for 48.5 deaths per 1,000,000 prescriptions (Morgan et al., 2004). By contrast, over the same period SSRIs accounted for around 6% of deaths by suicide, and other antidepressants, including venlafaxine, accounted for around 3%. This is equivalent to 1 and 5.2 deaths per 1,000,000 prescriptions, respectively (Morgan et al., 2004). Venlafaxine accounted for 8.5 deaths per 1,000,000 prescriptions. Morgan and colleagues (2004) showed an overall reduction in mortality rates over the time period studied, with a fall in rates related to TCAs, little change for SSRIs, but an increase for other antidepressants largely due to venlafaxine. It should be noted that the MHRA (2006) concluded that the increased rate seen with venlafaxine was partly, but not wholly, attributable to individual characteristics.
Adapting existing NICE guideline recommendations
The GDG considered the evidence concerning side effects and related issues in Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults (NCCMH, 2011b; NICE, 2011c). After careful consideration they identified two areas of particular importance, but the related recommendations required some adaptation for use in the current guideline (see Chapter 3 for an explanation of the method). These recommendations are set out in Table 14. The rationale for why recommendations were adapted is explained in the right-hand column of the table. In column one the numbers refer to the recommendations in Generalised Anxiety Disorder and Panic Disorder. In column two the numbers in brackets following the recommendation refer to Section 6.13 in this guideline.
Table 14
Recommendations from Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults for inclusion.
Clinical summary
Previous NICE guidelines support the view of the GDG that the side-effect profile of the various pharmacological interventions that could potentially be used in social anxiety disorder are common to many disorders. In particular, nausea, insomnia and sexual dysfunction associated with the SSRIs and SNRIs fitted with their experience of the use of such treatments in social anxiety disorder. The GDG saw no reason not to take into account the wide range of side effects concerning the cardiovascular system and the problems with sedation, tolerance, withdrawal and potential dependence associated with the use of benzodiazepines. Similarly, the GDG noted suicidality and discontinuation symptoms as problems associated with antidepressant drug use in general, and the risk of gastrointestinal bleeding associated with the use of SSRIs.
6.7. PSYCHOLOGICAL INTERVENTIONS
6.7.1. Cognitive behavioural interventions – individual
Fifteen trials (CLARK2003, CLARK2006, CLARK2012, COTTRAUX2000, EMMELKAMP2006, GOLDIN2012, HERBERT2004, MORTBERG2007, LEICHSENRING2012, LEDLEY2009, OOSTERBAAN2001, PRASKO2003, ROBILLARD2010, STANGIER2003, STANGIER2011) evaluated individual CBT/CT and were included in the NMA (562 participants on treatment). At post-treatment, there was a large effect for the class compared with waitlist (SMDN = −1.19, 95% CrI = −1.57 to −0.81); this was the only group of interventions (psychological or pharmacological) that differed significantly from both waitlist and pill placebo. The content, number of sessions and duration of treatment varied across trials; interventions were grouped into categories based on these features.
Compared with waitlist, one trial (STANGIER2003; 18 participants on treatment) reported a non-significant effect on symptoms of social anxiety at follow-up (SMD = −0.60, 95% CI = −1.26 to 0.05). One trial (LEDLEY2009; 15 participants on treatment) reported a non-significant effect on quality of life (SMD = −0.40, 95% CI = −1.08 to 0.29). In six trials (CLARK2006, CLARK2012, LEICHSENRING2012, ROBILLARD2010, STANGIER2003, STANGIER2011; 307 participants on treatment), there was a large effect on symptoms of depression at post-treatment (SMD = −0.86, 95% CI = −1.17 to −0.54) with substantial heterogeneity between trials (I 2 = 52%, Chi2 = 14.61, p = 0.04) and between subgroups (I2 = 82%, Chi2 = 10.94, p = 0.004). In one trial (STANGIER2003; 18 participants on treatment), the effect was not statistically significant for depression at follow-up (SMD = −0.51, 95% CI = −1.15 to 0.14). In three trials (CLARK2012, LEDLEY2009, STANGIER2003; 92 participants on treatment), there was a large effect on anxiety-related disability at post-treatment (SMD = −1.23, 95% CI = −2.08 to −0.37) with considerable heterogeneity (I 2 = 83%, Chi2 = 17.21, p = 0.0006). In one trial (STANGIER2003; 18 participants on treatment), there was no evidence of an effect on disability at follow-up (SMD = −0.35, 95% CI = −0.99 to 0.29).
Specific forms of individual CBT/CT
Two trials (GOLDIN2012, LEDLEY2009) included CBT (53 participants on treatment) delivered following the Heimberg manual (Hope et al., 2006) compared with waitlist. Participants received approximately 16 hours of therapy over 16 to 20 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −1.02, 95% CrI = −1.42 to −0.62).
Three trials (CLARK2003, CLARK2006, CLARK2012) included CT (97 participants on treatment) delivered following the Clark and Wells (1995) manual compared with waitlist, pill placebo, fluoxetine and exposure. Participants received approximately 21 hours of therapy over 14 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −1.56, 95% CrI = −1.85 to −1.27).
Six trials included one or more groups receiving a form of individual CBT that did not appear to follow one of the manuals above (COTTRAUX2000, EMMELKAMP2006, HERBERT2004, OOSTERBAAN2001, ROBILLARD2010, PRASKO2003; 164 participants on treatment) compared with waitlist, moclobemide, psychodynamic psychotherapy, supportive therapy, and another form of individual CBT. Participants received approximately 10 to 30 hours of therapy over 12 to 26 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −1.19, 95% CrI = −1.48 to −0.89).
Four trials (LEICHSENRING2012, MORTBERG2007, STANGIER2003, STANGIER2011) included CT (shortened sessions) with reduced therapist time for behavioural experiments (249 participants on treatment) compared with waitlist, group CBT, IPT and psychodynamic psychotherapy. Participants received approximately 15 hours of therapy over 15 to 26 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.97, 95% CrI = −1.21 to −0.73).
One trial (RENNER2008) reported that participants received individual CBT (14 participants on treatment) or applied relaxation (14 participants on treatment), but the intervention was not sufficiently described to determine whether it was similar to other interventions in the analysis, so a separate pairwise analysis was conducted. Comparing two sessions of a poorly described CBT intervention with two sessions of applied relaxation, there was a large effect favouring applied relaxation at post-treatment (SMD = 1.13, 95% CI = 0.32 to 1.94).
6.7.2. Cognitive behavioural interventions – group
Twenty seven trials (ALDEN2011, ANDREWS2011, BLANCO2010, BJORNSSON-2011, BORGEAT2009, DAVIDSON2004b, FURMARK2002, GELERNTER1991, GRUBER2001, HEDMAN2011, HEIMBERG1990, HEIMBERG1998, HERBERT2005, HOPE1995, KOSZYCKI2007, MATTICK1988, MATTICK1989, MCEVOY2009, MORGAN1999, MORTBERG2007, OTTO2000, PIET2010, RAPEE2007, RAPEE2009, SALABERRIA1998, STANGIER2003, WONG2006) evaluated group CBT and were included in the NMA (984 participants on treatment). At post-treatment, there was a large effect for the class compared with waitlist (SMDN = −0.92, 95% CrI = −1.34 to −0.51). In two trials (SALABERRIA1998, STANGIER2003; 39 participants on treatment), the effect was not statistically significant for symptoms of social anxiety at follow-up (SMD = −0.76, 95% CI = −1.98 to 0.47) compared with waitlist, with substantial heterogeneity between trials (I2 = 85%; Chi2 = 6.80, p = 0.009). In one trial (HEIMBERG1990; 15 participants on treatment), there was no evidence of an effect on symptoms of social anxiety disorder at follow-up (SMD = −0.37, 95% CI = −1.14 to 0.39) compared with psychological placebo.
In two trials (GRUBER2001, STANGIER2003; 51 participants on treatment), the effect was not statistically significant for depression compared with waitlist at post-treatment (SMD = −0.58, 95% CI = −1.24 to 0.08) with substantial heterogeneity (I2 = 63%, Chi2 = 5.43, p = 0.07). In two trials (SALABERRIA1998, STANGIER2003; 39 participants on treatment) there was a medium effect (SMD = −0.59, 95% CI = −1.04 to −0.14) at follow-up, with no heterogeneity (I2 = 0%, Chi2 = 0.78, p = 0.38). In two trials (HEIMBERG1990, HEIMBERG1998; 48 participants on treatment), there was no evidence of an effect on depression compared with psychological placebo at post-treatment (SMD = 0.15, 95% CI = −0.81 to 1.11), with considerable heterogeneity (I2 = 81%, Chi2 = 5.31, p = 0.02). In one trial (HEIMBERG1990; 27 participants on treatment), there was no evidence of an effect at follow-up (SMD = −0.23, 95% CI = −0.85 to 0.39), with no significant heterogeneity (I2 = 20%, Chi2 = 1.25, p = 0.26). One trial (STANGIER2003) comparing group CBT (22 participants on treatment) with waitlist reported no evidence of an effect on anxiety-related disability at post-treatment (SMD = −0.15, 95% CI = −0.75 to 0.45) or at follow-up (SMD = −0.44, 95% CI = −1.06 to 0.18). A trial with two CBT groups (RAPEE2009; 127 participants on treatment) reported a small effect compared with psychological placebo at post-treatment (SMD = −0.35, 95% CI = −0.67 to −0.03) with no heterogeneity between the groups (I2 = 0%, Chi2 = 0.53, p = 0.47). None of the trials reported quality of life outcomes.
In addition to the trials of acute treatment, one trial (HEIMBERG2012) randomised participants to paroxetine alone or CBT plus paroxetine after an open-label phase of the drug for 12 weeks. During the randomised phase, participants in the combination therapy group (32 participants on treatment) received 16 weeks of group CBT alongside paroxetine (unknown dosage). Because of the open-label phase, the GDG chose not to include the trial in the NMA. At post-treatment, there was a small effect in favour of combination therapy on symptoms of social anxiety, which was nearly significant (SMD = −0.49, 95% CI = −1.00 to 0.02).
Specific forms of group CBT
Eleven trials (BLANCO2010, GELERNTER1991, GRUBER2001, HEDMAN2011, HEIMBERG1990, HEIMBERG1998, HERBERT2005, HOPE1995, KOSZYCKI2007, OTTO2000, WONG2006) included group CBT (338 participants on treatment) delivered following Heimberg and colleagues' manual (Heimberg et al., 1995) compared with waitlist, pill placebo, psychological placebo, alprazolam, clonazepam, exposure, group CBT with phenelzine, mindfulness training and phenelzine. Participants received between 20 and 30 hours of therapy in groups of about seven people over 12 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.80, 95% CrI = −1.02 to −0.58).
Sixteen trials (ALDEN2011, ANDREWS2011, BJORNSSON2011, BORGEAT2009, DAVIDSON2004b, FURMARK2002, MATTICK1988, MATTICK1989, MCEVOY2009, MORGAN1999, MORTBERG2007, PIET2010, RAPEE2007, RAPEE2009, SALABERRIA1998, STANGIER2003) included one or more groups receiving a form of group CBT that did not appear to follow Heimberg and colleagues' manual (583 participants on treatment) compared with waitlist, pill placebo, psychological placebo, citalopram, exposure, fluoxetine, group CBT with fluoxetine, individual CT, mindfulness training, self-help, treatment as usual, and another form of group CBT. Participants received approximately 6 to 14 hours of therapy over 7 to 15 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.85, 95% CrI = −1.04 to −0.67).
One trial (RAPEE2009) also used an enhanced form of group CBT with enhanced exposure (63 participants on treatment) and there was a large effect compared with waitlist (SMDN = −1.10, 95% CrI = −1.49 to −0.71).
6.7.3. Cognitive bias modification
Seven trials (AMIR2009, AMIR2012, BEARD2011, BOETTCHER2011, CARLBRING2012, HEEREN2012, SCHMIDT2009) compared computerised cognitive bias modification (156 participants on treatment) with a sham intervention. Studies lasted 4 days to 6 weeks, with total time using the programs ranging from 4 to 21 hours. No trials included an intervention connected to the NMA, so pairwise comparisons were performed for all relevant outcomes.
In three trials (AMIR2012, BOETTCHER2011, SCHMIDT2009; 75 participants on treatment), there was no evidence of an effect on recovery at post-treatment (RR = 0.59, 95% CI = 0.25 to 1.42), with considerable heterogeneity (I2 = 92%, Chi2 = 23.71, p = 0.00001). One trial (SCHMIDT2009; 19 participants on treatment) reported a moderate effect at follow-up (RR = 0.62, 95% CI = 0.39 to 0.99).
Combining all seven trials (AMIR2009, AMIR2012, BEARD2011, BOETTCHER2011, CARLBRING2012, HEEREN2012, SCHMIDT2009; 156 participants on treatment), there was moderate-quality evidence of a modest effect on continuous measures of social anxiety at post-treatment (SMD = −0.30, 95% CI = −0.55 to −0.05; I2 = 27%, Chi2 = 8.26, p = 0.22). At follow-up, there was low-quality evidence and the effect was not statistically significant (SMD = −0.58, 95% CI = −1.20 to 0.04), with considerable heterogeneity (I2 = 79%, Chi2 = 14.13, p = 0.003).
One trial (CARLBRING2012; 40 participants on treatment) reported no evidence of an effect on quality of life at post-treatment (SMD = −0.20; 95% CI = −0.64 to 0.24) or at follow-up (SMD = −0.16, 95% CI = −0.60 to 0.28). In four trials (AMIR2009, AMIR2012, BOETTCHER2011, SCHMIDT2009), there was no evidence of an effect on depression at post-treatment (SMD = 0.04, 95% CI = −0.43 to 0.51), with substantial heterogeneity (I2 = 64%, Chi2 = 8.44, p = 0.04). In two trials (BOETTCHER2011, SCHMIDT2009; 53 participants on treatment), there was no evidence of an effect on depression at follow-up (SMD = −0.03, 95% CI = −0.64 to 0.59), with no significant heterogeneity (I2 = 47%, Chi2 = 1.88, p = 0.17). In two trials (AMIR2009, AMIR2012; 45 participants on treatment), there was a medium effect on anxiety-related disability at post-treatment (SMD = −0.61, 95% CI = −1.03 to −0.19) with no heterogeneity.
6.7.4. Exercise
One trial (JAZAIERI2012) compared an exercise intervention (18 participants on treatment) with mindfulness-based stress reduction. Participants were required to complete at least two individual moderate intensity exercise sessions and one group session per week for 8 weeks. At post-treatment, there was no evidence of an effect compared with waitlist (SMDN = −0.34, 95% CrI = −1.06 to 0.38).
6.7.5. Exposure in vivo and social skills training
Eight trials (ANDERSSON2006, BORGEAT2009, CLARK2006, HOPE1995, MATTICK1988, SALABERRIA1998, SMITS2006, STRAVYNSKI2000) reported a controlled effect for exposure or social skills training. At post-treatment, there was a large effect for the intervention class compared with waitlist (SMDN = −0.86, 95% CrI = −1.42 to −0.30).
All eight trials included one or more groups receiving exposure (199 participants on treatment) compared with waitlist, psychological placebo, group CBT, individual CT, social skills training and other forms of exposure. Participants received approximately 4 to 21 hours of therapy in groups over 1 to 14 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.83, 95% CrI = −1.07 to −0.59).
Three trials included social skills training, but two did not report usable outcomes: SHAW1979 (Shaw, 1979), ALDEN1989 (Alden, 1989). One trial (STRAVYNSKI2000) compared social skills training (32 participants on treatment) with exposure. Participants received 24 hours of therapy over 12 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.88, 95% CrI = −1.38 to −0.38).
In a pairwise analysis compared with waitlist, one trial (ANDERSSON2006) reported a medium effect of exposure (30 participants on treatment) on quality of life at post-treatment (SMD = −0.73, 95% CI = −1.25 to −0.22). In two trials (ANDERSSON2006, CLARK2006; 51 participants on treatment) compared with waitlist, there was a large effect of exposure on depression at post-treatment (SMD = −0.50, 95% CI = −0.89 to −0.10) with no heterogeneity (I2 = 0%, Chi2 = 0.97, p = 0.32). One trial (SALABERRIA1998) reported a large effect of exposure (18 participants on treatment) on depression at follow-up (SMD = −1.17, 95% CI = −1.87 to −0.48). None of the trials reported anxiety-related disability outcomes at any timepoint.
6.7.6. Exposure with cognitive enhancers
In four trials (GUASTELLA2008, GUASTELLA2009, HOFMANN2006, HOFMANN2012), participants received some exposure therapy and either a cognitive enhancer or pill placebo. The trials were considered to be different from those in the NMA because the exposure was a diminished form of what was provided in the other trials in the network. Pairwise comparisons were therefore performed.
Three trials (GUASTELLA2008, HOFMANN2006, HOFMANN2012) assigned participants to exposure with the cognitive enhancer D-cycloserine (127 participants on treatment) or exposure alone. There was a small effect on symptoms of social anxiety at post-treatment (SMD = −0.36, 95% CI = −0.61 to −0.11) with no heterogeneity (I2 = 0%, Chi2 = 0.47, p = 0.79). There was a small but not significant effect at follow-up (SMD = −0.20, 95% CI = −0.45 to 0.05) with no significant heterogeneity (I2 = 1%, Chi2 = 2.02, p = 0.36).
In one trial of oxytocin (GUASTELLA2009; 12 participants on treatment), there was no evidence of an effect on symptoms of social anxiety disorder at post-treatment (SMD = 0.26, 95% CI = −0.53 to 1.35) or at 1 month's follow-up (SMD = 0.15, 95% CI = −0.64 to 0.93).
6.7.7. Interpersonal psychotherapy
Two trials (LIPSITZ2008, STANGIER2011) of IPT (64 participants on treatment) compared with waitlist, individual CT and supportive therapy were included in the NMA. Participants received approximately 14 hours of therapy over 14 to 20 weeks. At post-treatment, there was evidence of a significant medium effect compared with waitlist (SMDN = −0.43, 95% CrI = −0.83 to −0.03).
6.7.8. Mindfulness training
Three trials (JAZAIERI2012, KOSZYCKI2007, PIET2010) included mindfulness training (64 participants on treatment) compared with exercise and group CBT. Participants received about 20 hours of therapy delivered in groups of approximately 12 people over 8 weeks. At post-treatment, there was evidence of a non-significant medium effect compared with waitlist (SMDN = −0.39, 95% CrI = −0.82 to 0.04).
6.7.9. Short-term psychodynamic psychotherapy
Three trials (EMMELKAMP2006, KNIJNIK2004, LEICHSENRING2012) included psychodynamic psychotherapy (185 participants on treatment) compared with waitlist, individual CT, individual CBT and supportive therapy. In the largest study (LEICHSENRING2012), which accounts for most of the reported effects, participants received approximately 1 hour of therapy per week for 26 weeks. At post-treatment, there was a medium effect compared with waitlist (SMDN = −0.62, 95% CrI = −0.93 to −0.31).
In a pairwise analysis compared with waitlist, one trial (LEICHSENRING2012; 132 participants on treatment) reported a small effect on depression at post-treatment (SMD = −0.39, 95% CI = −0.72 to −0.06). No trials reported controlled effects for symptoms at follow-up, quality of life or anxiety-related disability.
6.7.10. Supportive therapy
Two trials (COTTRAUX2000, LIPSITZ2008) compared supportive therapy (54 participants on treatment) with individual CBT and IPT. Participants received 3 and 14 hours of therapy over 12 and 14 weeks, respectively. At post-treatment there was no evidence of an effect compared with waitlist (SMDN = −0.26, 95% CrI = −0.72 to 0.21).
6.7.11. Self-help with and without support
Sixteen trials (ABRAMOWITZ2009, ANDERSSON2012, ANDREWS2011, BERGER2009, CARLBRING2007, CHUNG2008, FURMARK2009a, FURMARK2009b, HEDMAN2011, RAPEE2007, TITOV2008a, TITOV2008b, TITOV2008c, TITOV2009a, TITOV2009b, TITOV2010b) evaluated self-help with or without support (1,154 participants on treatment) and were included in the NMA. All trials used a cognitive behavioural approach and included varying levels of contact with researchers and therapists. At post-treatment, there was a medium effect for self-help without support (406 participants on treatment) compared with waitlist (SMDN = −0.75, 95% CrI = −1.25 to −0.25) and a large effect for self-help with support (748 participants on treatment) compared with waitlist (SMDN = −0.86, 95% CrI = −1.36 to −0.37).
In a pairwise analysis compared with waitlist, one trial (FURMARK2009a; 80 participants on treatment) reported no evidence of an effect on recovery at follow-up (RR = 0.77, 95% CI = 0.56 to 1.06). In a pairwise analysis of three trials (ANDERSSON2012, CARLBRING2007, FURMARK2009a; 211 participants on treatment) compared with waitlist, there was a medium effect on quality of life at post-treatment (SMD = −0.51, 95% CI = −0.86 to −0.17) with substantial heterogeneity between trials (I2 = 55%, Chi2 = 6.70, p = 0.08) and between subgroups that varied by contact (I2 = 84.2%, Chi2 = 6.35, p = 0.01). In one trial (FURMARK2009a; 80 participants on treatment), the effect was not statistically significant for quality of life at follow-up (SMD = −0.32, 95% CI = −0.70 to 0.06) and there was no heterogeneity. In a pairwise analysis of six trials (ABRAMOWITZ2009, ANDERSSON2012, BERGER2009, CARLBRING2007, FURMARK2009a, TITOV2008c; 314 participants on treatment) compared with waitlist, there was a medium effect on depression at post-treatment (SMD = −0.61, 95% CI = −0.78 to −0.43), with no heterogeneity between trials and no significant heterogeneity between subgroups (I2 = 20%, Chi2 = 3.74, p = 0.29). In one trial (FURMARK2009a; 80 participants on treatment), the effect was not statistically significant for depression at follow-up (SMD = −0.22, 95% CI = −0.60 to 0.16). In a pairwise analysis of two trials (BERGER2009, TITOV2008c; 92 participants on treatment) compared with waitlist, the effect was not statistically significant for anxiety-related disability (SMD = −0.32, 95% CI = −0.66 to 0.02) with no heterogeneity.
Self-help without support
Three trials (TITOV2008c, TITOV2009b, TITOV2010b) compared internet self-help (270 participants on treatment) with waitlist and self-help with support. At post-treatment, there was a medium effect compared with waitlist (SMDN = −0.66, 95% CrI = −0.94 to −0.39).
Four trials (CHUNG2008, FURMARK2009a, FURMARK2009b, RAPEE2007) compared a self-help book (136 participants on treatment) with waitlist, group CBT, internet self-help without support, and self-help with support. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.84, 95% CrI = −1.08 to −0.60).
Self-help with support
Twelve trials (ANDERSSON2012, ANDREWS2011, BERGER2009, CARLBRING2007, FURMARK2009a, FURMARK2009b, HEDMAN2011, TITOV2008a, TITOV2008b, TITOV2008c, TITOV2009a, TITOV2009b) compared internet self-help with support (696 participants on treatment) with waitlist, group CBT, self-help without support, and another form of internet self-help with support. Contact with a researcher or therapist varied, but usually included 2 to 3 hours of contact during treatment (by email or telephone) in addition to an initial clinical assessment. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.88, 95% CrI = −1.04 to −0.71).
Two trials (ABRAMOWITZ2009, CHUNG2008) compared a self-help book with support (52 participants on treatment) with waitlist and self-help without support. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.87, 95% CrI = −1.33 to −0.40). Additionally, one trial compared a self-help book with a moderated discussion group (28 participants) with other forms of self-help. At post-treatment, there was a large effect compared with waitlist (SMDN = −0.85, 95% CrI = −1.17 to −0.53).
6.8. COMBINED PSYCHOLOGICAL AND PHARMACOLOGICAL INTERVENTIONS
One trial (DAVIDSON2004b) compared combination therapy (group CBT combined with fluoxetine) with fluoxetine alone, group CBT alone, pill placebo, and group CBT with pill placebo. Participants (59 participants on treatment) received 14 hours of group CBT and 47 mg of fluoxetine daily for 14 weeks. At post-treatment, there was a medium effect for combination therapy compared with waitlist (SMDN = −0.95, 95% CrI = −1.33 to −0.57).
One trial (BLANCO2010) compared combination therapy (Heimberg's group CBT combined with phenelzine) with phenelzine alone, group CBT alone, and pill placebo. Participants (32 participants on treatment) received 30 hours of group CBT and 62 mg of phenelzine daily for 12 weeks. At post-treatment, there was a large effect compared with waitlist (SMDN = −1.69, 95% CrI = −2.10 to −1.27).
One trial (PRASKO2003) compared combination therapy (group CBT combined with moclobemide) with moclobemide alone and individual CBT with pill placebo. There were 22 participants receiving combination therapy, the dose of which was not reported. At post-treatment there was a large effect compared with waitlist (SMDN = −1.23, 95% CrI = −1.72 to −0.74).
One trial (KNIJNIK2008) compared combination therapy (psychodynamic psychotherapy combined with clonazepam) with clonazepam alone. Participants (29 participants on treatment) received 18 hours of psychodynamic psychotherapy and 1 mg of clonazepam daily for 12 weeks. At post-treatment there was a large effect compared with waitlist (SMDN = −1.28, 95% CrI = −1.82 to −0.75).
One trial (CRASKE2011) compared preference-based therapy (74 participants on treatment) with treatment as usual. There was a medium effect on symptoms of social anxiety disorder at post-treatment (SMD = −0.48, 95% CI = −0.83 to −0.14) and at 12-month follow-up (SMD = 0.39, 95% CI = −0.74 to −0.05), which was no longer significant after 18 months (SMD = 0.30, 95% CI = −0.64 to 0.05).
6.9. SPECIFIC SUBGROUPS
6.9.1. Interventions for fear of public speaking
One trial (NEWMAN1994) compared exposure (16 participants on treatment) with waitlist for people with social anxiety disorder and a predominant fear of public speaking. Participants received approximately 16 hours of therapy in groups of six people over 8 weeks. At post-treatment, there was a non-significant medium effect on symptoms of social anxiety disorder (SMD = −0.60, 95% CI = −1.30 to 0.11).
In one trial (TILLFORS2008) participants with social anxiety disorder and a predominant fear of public speaking received self-help and either five sessions of exposure (18 participants on treatment) or email support from the therapist over 9 weeks. There was no difference between the groups on symptoms of social anxiety disorder at post-treatment (SMD = −0.10, 95% CI = −0.74 to 0.54) or at follow-up (SMD = 0.15, 95% CI = −0.51 to 0.81).
One trial (BOTELLA2010) compared individual CBT (36 participants on treatment) with self-help and waitlist for participants with a predominant fear of public speaking. At post-treatment, there were large effects compared with waitlist on symptoms of social anxiety disorder for both CBT (SMD = −1.18, 95% CI = −1.72 to −0.65) and self-help (SMD = −1.09, 95% CI = −1.56 to −0.63).
6.9.2. Interventions for fear of blushing, trembling or sweating
One trial (MULKENS2001) compared exposure (12 participants on treatment) with attention training for people with social anxiety disorder and a predominant fear of blushing. Participants received 6 hours of exposure therapy or attention training over 6 weeks. There was no evidence of an effect on symptoms of social anxiety at post-treatment (SMD = −0.42, 95% CI = −1.20 to 0.36) or at follow-up (SMD = −0.15, 95% CI = −1.02 to 0.71).
One trial (BOGELS2006) compared task concentration training (33 participants on treatment) with applied relaxation (32 participants on treatment) for people with social anxiety disorder and a predominant fear of blushing, trembling or sweating. Participants received approximately 13 hours of attention training or applied relaxation therapy over 8 weeks. There was no difference on symptoms of social anxiety disorder at post-treatment (SMD = 0.01, 95% CI = −0.48 to 0.50) or at 3-month (SMD = 0.02, 95% CI = −0.47 to 0.50) or 12-month follow-up (SMD = −0.17, 95% CI = −0.65 to 0.32).
One trial (BOGELS2008) compared social skills training (28 participants on treatment) with group CBT (27 participants on treatment) for people with social anxiety disorder and a predominant fear of blushing, trembling or sweating. Participants received 24 hours of CBT or social skills training in groups of seven people over 12 weeks. There was no evidence of an effect on symptoms of social anxiety disorder at post-treatment (SMD = 0.19, 95% CI = −0.34 to 0.72) or at 12-month follow-up (SMD = 0.11, 95% CI = −0.42 to 0.64).
6.9.3. Physical interventions for fear of blushing or sweating
One trial (CONNOR2004) randomised participants with social anxiety and palmar hyperhidrosis (excessive hand sweating) to paroxetine with botulinum toxin injections (20 participants on treatment) or paroxetine with placebo injections. There was no evidence of a differential effect on symptoms of social anxiety disorder at post-treatment (SMD = −0.22; 95% CI = −0.84 to 0.41) and the between-group effect for anxiety-related disability was a non-significant medium effect (SMD = −0.63; 95% CI = −1.27 to 0.02).
Systematic searches did not identify any trials of thoracic sympathectomy for the treatment of people with social anxiety disorder.
In the absence of evidence about physical interventions for people with social anxiety disorder, the GDG considered extrapolating from trials that suggested physical interventions may reduce blushing or sweating (for example, in people with hyperhidrosis [Boley et al., 2007]). As social anxiety disorder is characterised by fear and avoidance of situations in which the person believes something embarrassing may happen rather than the actual presence of physical symptoms, the GDG agreed that the results from other populations were not relevant and could not be extrapolated to this guideline.
6.9.4. Residential interventions
One trial (BORGE2008) compared group CBT (35 participants on treatment) with IPT (38 participants on treatment) for people with social anxiety receiving residential treatment. Participants received four group sessions of around 45 minutes and one individual session per week of either IPT or CBT for 10 weeks. There was no difference between groups on symptoms of social anxiety disorder at post-treatment (SMD = −0.07, 95% CI = −0.53 to 0.39) or at follow-up (SMD = −0.02, 95% CI = −0.48 to 0.44).
6.9.5. Interventions for social anxiety disorder and comorbid alcohol misuse
Two trials (BOOK2008, RANDALL2001a) compared paroxetine (26 participants on treatment) with placebo for people with social anxiety disorder and comorbid alcohol misuse or dependence. Participants received 45 mg daily for 8 and 16 weeks. There was a large effect on symptoms of social anxiety disorder at post-treatment (SMD = −0.91, 95% CI = −1.56 to −0.26) with no significant heterogeneity (I2 = 15%, Chi2 = 1.18, p = 0.28). There was no significant effect on withdrawal because of side effects (RR = 3.29, 95% CI = 0.14 to 76.33).
Three trials (HAYES2006, HEIDEMAN2008 [Heideman, 2008], RANDALL2001b [Randall et al., 2001b]) included a CBT intervention for people with social anxiety disorder and comorbid alcohol misuse, but two of these did not report usable data for symptoms of social anxiety disorder. In the remaining trial (HAYES2006), all participants received CBT and one group also received an intervention for alcohol misuse (ten participants on treatment). There was no difference between groups on symptoms of social anxiety disorder at post-treatment (SMD = −0.32, 95% CI = −1.15 to 0.51).
6.9.6. Interventions for social anxiety disorder comorbid with attention deficit hyperactivity disorder
One trial (ADLER2009) compared atomoxetine (200 participants on treatment) with placebo for people with comorbid social anxiety disorder and ADHD. Participants received 83 mg daily for 14 weeks. There was a small effect on symptoms of social anxiety disorder at post-treatment (SMD = −0.24, 95% CI = −0.44 to −0.04) and there was a small effect on the number of people reporting any adverse event (RR = 1.09, 95% CI = 1.00 to 1.19).
6.10. HEALTH ECONOMIC EVIDENCE
6.10.1. Systematic literature review
The systematic search of the economic literature undertaken for this guideline identified four eligible studies on interventions for adults with social anxiety (François et al., 2008; Gould et al., 1997; Hedman et al., 2011a; Titov et al., 2009b). One study was conducted in the UK (François et al., 2008), one in the US (Gould et al., 1997), one in Sweden (Hedman et al., 2011a) and one in Australia (Titov et al., 2009b). Details on the methods used for the systematic review of the economic literature are described in Chapter 3; completed methodology checklists of the studies are provided in Appendix 21, and the respective evidence tables are provided in Appendix 22.
François and colleagues (2008) assessed the cost effectiveness of escitalopram versus placebo in maintenance treatment of adults with social anxiety who had previously responded to treatment with escitalopram, from a UK NHS and personal social services (PSS) perspective, as well as from a societal perspective. The economic analysis was conducted alongside a multi-national placebo-controlled trial of escitalopram for relapse prevention (MONTGOMERY2005). The study sample consisted of people with a primary diagnosis of social anxiety disorder who had responded to 12 weeks of open-label treatment with escitalopram. Treatment was continued for 24 weeks unless a person relapsed or was withdrawn for other reasons. Costs considered in the analysis included physician consultations and other healthcare professional visits, hospitalisation and drug acquisition costs; productivity costs were reported separately. The cost year was 2006. The primary outcome of the analysis was the HRQoL of study participants, measured by Short Form Questionnaire Six Dimensional Health State Classification (SF-6D) utility scores (Brazier et al., 2002).
Costs were reported exclusively for people who did not relapse during the trial. The cost per person not relapsing was £111 in the escitalopram arm and £180 in the placebo arm over the first 12 weeks of the trial (p = 0.39), while the respective figures over the period from 12 to 24 weeks of the trial were £124 and £202 (p = 0.44). Escitalopram led to a reduction of relapses compared with placebo. The mean SF-6D scores at the end of the trial (24 weeks) were 0.715 for escitalopram and 0.698 for placebo (p = 0.009). Based on these results, the authors concluded that escitalopram was an effective treatment that led to significant improvement in HRQoL and resulted in cost savings that might potentially offset drug acquisition costs.
The study by François and colleagues (2008) is directly applicable to the guideline context as it is conducted from the NHS and PSS perspective. The measure of outcome was reported in the form of utility scores that were not transformed into quality-adjusted life years (QALYs); nevertheless, this did not affect interpretation of the results given that escitalopram was the dominant option. One of the limitations of the study was that costs were reported exclusively for people not relapsing during the trial; costs incurred by people who relapsed were not included in the analysis. However, given that escitalopram led to a reduction of relapses in the trial, omission of costs for people relapsing, which are expected to be higher than those incurred by ‘non-relapsed’ participants, is likely to only have underestimated the cost savings associated with escitalopram. The authors acknowledged a number of other limitations in how their study was conducted, such as the fact that in the analysis it was not possible to distinguish between study participants who did not utilise any resource and participants who failed to report resource use; this may have led to an underestimation of costs, irrespective of treatment group or time period of the analysis. Moreover, costs were estimated by applying UK unit prices to resource use reported from study participants in other countries; however, treatment may have a different impact on resource utilisation across countries in terms of type and frequency of resources used, and it was not possible to account for this in the study. Overall, the study findings suggest that escitalopram may be a cost-effective option in the maintenance treatment of adults with social anxiety.
Gould and colleagues (1997) evaluated the cost effectiveness of group CBT relative to pharmacological treatment (comprising phenelzine, fluvoxamine or clonazepam) and to combination therapy (comprising group CBT and pharmacological treatment) for adults with social anxiety disorder in the US. For each therapy considered in the study, the authors estimated its intervention cost over 2 years of treatment, and assessed its effect size for symptoms of social anxiety disorder or avoidance versus a control (mainly a minimal intervention: placebo or waitlist) after conducting a systematic review and meta-analysis of published trials. Intervention costs were estimated from a third-party payer perspective and consisted of CBT sessions including booster sessions, as well as drug acquisition costs, prescription charges and consultations with clinicians for pharmacological interventions.
The total intervention cost was estimated at $760 for group CBT; for pharmacological treatment it ranged from $1,744 (clonazepam) to $5,496 (fluvoxamine); and for combination therapy it ranged from $2,504 to $6,256 (price year not reported, but it was likely to have been 1996). The effect size was found to be 0.74 for group CBT, 0.62 for pharmacological treatment and 0.49 for combination therapy. Based on these findings, the authors concluded that group CBT was the most cost-effective treatment option for adults with social anxiety disorder in the US because it had the highest effect size and the lowest intervention cost.
The study is only partially applicable to the UK setting because it was conducted from a third-party payer perspective in the US, and suffers from a number of serious methodological limitations (including that costs only included intervention costs and other healthcare costs incurred by people with social anxiety disorder were not considered). More importantly, the estimates of effect size for each intervention referred to a different comparator (baseline treatment): this was, for example, waitlist or minimal treatment for group CBT and placebo for pharmacological treatment. Placebo is likely to have a significant relative effect compared with waitlist, which means that the effectiveness of pharmacological treatment is likely to have been underestimated relative to group CBT. The figures for effect sizes reported for each intervention were not comparable and should not be used to assess comparative effectiveness; instead, a (direct or indirect) relative effect size between the treatments considered in the study should have been estimated to assess comparative effectiveness. Finally, the uncertainty around the cost estimates and effect sizes was not reported. The findings of this study should be therefore interpreted with caution.
Hedman and colleagues (2011a) explored the cost effectiveness of computer-based self-help with support relative to group CBT for adults with social anxiety disorder in Sweden. The economic analysis, which was performed alongside an RCT (HEDMAN2011), adopted a societal perspective; nevertheless, medical costs were reported separately. Costs included intervention costs (therapist's time), GP visits, consultations with doctors, counsellors, psychotherapists, medical specialists and physiotherapists, health-related services (for example, alternative and home care, self-help groups), as well as productivity losses including informal care. The primary measure of outcome was the clinician-administered LSAS; in addition, the study estimated the percentage of responders defined using the Jacobson and Truax (1991) criteria. HRQoL was also measured for each participant, using the European Quality of Life – 5 Dimensions (EQ-5D) UK tariff utility scores. Costs and outcomes were measured at post-treatment (15 weeks) and at 6 months' follow-up.
Total mean medical costs over the 15 weeks of treatment reached $1,343 per person for self-help with support and $3,502 per person for group CBT (in 2009 US$); of these, $464 and $2,687 comprised intervention costs of self-help with support and group CBT, respectively. The total mean medical costs over the period from 15 weeks until 6 months' follow-up were $1,067 and $841 per person, for self-help with support and group CBT, respectively. In terms of outcomes, at 15 weeks the mean LSAS score was 39.4 (SD 19.9) for self-help with support and 48.5 (SD 25.0) for group CBT; the percentage of responders was 55% for self-help with support and 34% for group CBT; and the mean EQ-5D utility score was 0.82 (SD 0.14) for self-help with support and 0.80 (SD 0.17) for group CBT. At 6 months, the mean LSAS score was 32.1 (SD 23.1) versus 40.7 (SD 23.7) for self-help with support and group CBT, respectively; the percentage of responders was 64% versus 45%, while the mean EQ-5D utility score was 0.85 (SD 0.14) versus 0.81 (SD 0.17) for self-help with support and group CBT, respectively. Self-help with support showed lower intervention costs, which resulted in lower total medical costs, compared with group CBT, while the effectiveness of the two interventions was similar. Thus the study concluded that self-help with support was more cost effective than group CBT in adults with social anxiety disorder. The authors also performed probabilistic sensitivity analysis and reported that the probability of self-help being cost effective compared with group CBT was 81% at zero willingness to pay (WTP) per extra person responding to treatment, while this probability would rise at 89% at a WTP of $3,000 per extra person responding. The authors also reported that self-help with support had 80% probability of being cost effective at WTP ranging between zero and $40,000 per QALY gained.
The results of probabilistic analysis should be interpreted with caution because it appears that the authors double-counted the intervention costs (that is, they included them both in cost estimates during the 16 weeks of treatment and in cost estimates during the follow-up period). Moreover, although the study reports the probability of cost effectiveness for different levels of WTP per extra QALY gained, no QALYs seem to have been estimated in the study for each intervention; instead, EQ-5D utility scores were measured post-treatment and at 6-month follow-up. The study has not considered the costs associated with provision of computers or other infrastructure required in order to run the computerised self-help program. In any case, the study is only partially applicable to the guideline context because it was conducted in Sweden.
The study by Titov and colleagues (2009b) examined the cost effectiveness of computer-based self-help with support compared with group CBT for adults with social anxiety disorder from the perspective of the Australian health service. Costs included therapists' time only. The primary outcome measure was the number of years lived with disability (YLD) averted. Clinical effectiveness of the interventions assessed and related resource use were based on two RCTs (TITOV2008a, TITOV2008b) and a non-comparative study.
According to the study results, the mean cost of self-help with support was AU$300 per person, while the mean cost of group CBT reached AU$800 per person (2008 prices). The number of YLD averted of self-help with support versus waitlist was estimated at 0.2007; the number of YLD averted of group CBT compared with a ‘do nothing’ option was estimated at 0.1407. The authors estimated the incremental cost-effectiveness ratio (ICER) of self-help with support versus waitlist at AU$1,495 per YLD averted, and of group CBT versus a ‘do nothing’ option at AU$5,686 per YLD averted. Based on these findings they concluded that self-help with support was a more cost-effective option because it provided a better outcome at a lower cost.
The study is only partially applicable to the guideline context because it was conducted in Australia. Moreover, it is characterised by a number of important limitations. Cost estimates were limited to those relating to therapists' time. Costs of computers and other infrastructure required in order to run the computerised self-help program, as well as costs associated with further healthcare resource use (for example, visits to other healthcare professionals), were not considered. Also, it was not clear how effect sizes were estimated from different studies with different designs and then converted into number of YLD averted. There was no direct or indirect comparison between the two interventions assessed; rather, results were presented for each intervention in comparison with a given control (waitlist or do nothing).
Overall the existing economic evidence on interventions for adults with social anxiety disorder is sparse, not directly applicable to the guideline context, and characterised by serious methodological limitations. Based on this evidence, no safe conclusion on the cost effectiveness of the range of interventions available for adults with social anxiety disorder in the UK can be made.
6.10.2. Economic modelling
Introduction – objective of economic modelling
The cost effectiveness of interventions for adults with social anxiety disorder was considered by the GDG as an area with likely significant resource implications. Existing economic evidence in this area was very limited and not directly applicable to the UK setting: only one out of the four relevant economic studies identified in the guideline systematic review was conducted in the UK. The economic studies included in the review were characterised by several important limitations; moreover, they assessed only a limited number of interventions available in the UK for adults with social anxiety disorder. However, the clinical evidence was judged to be sufficient and of adequate quality to inform primary economic modelling. Based on the above considerations, this area was prioritised for further economic analysis. An economic model was therefore developed to assess the relative cost effectiveness across different interventions for adults with social anxiety disorder in the UK.
Economic modelling methods
Interventions assessed
The guideline economic analysis assessed those interventions for adults with social anxiety disorder that are available in the UK, and for which there was adequate clinical evidence to indicate their effectiveness along with an acceptable risk-to-benefit ratio. Further to these criteria, pharmacological interventions were included in the economic analysis if they are prescribed in routine UK clinical practice for the management of anxiety disorders. Benzodiazepines were not included in the economic analysis because they are not indicated for use longer than 2 to 4 weeks for the treatment of anxiety (Joint Formulary Committee, 2013). Computerised interventions were included in the economic analysis, despite their current unavailability in UK routine practice, because they are used in other countries and because they are likely to become available in the UK. Moreover, this guideline updates the NICE TA on computerised CBT (CCBT) for depression and anxiety (NICE, 2006), regarding phobias (see Chapter 8). Social skills training was not considered a separate intervention in the analysis because it is an element contained in other psychological interventions that were included in the economic model and the GDG was of the view that it is best provided as part of those therapies rather than as a separate intervention.
Based on the above criteria the following interventions were included in the economic analysis:
Pharmacological interventions: citalopram, escitalopram, fluoxetine, fluvoxamine, mirtazapine, moclobemide, paroxetine, phenelzine, pregabalin, sertraline and venlafaxine; for the latter, extended release formulations were considered, in accordance with the formulations used in the relevant RCTs included in the NMA that informed the economic model.
Psychological interventions: group CBT, individual CBT, group CBT (Heimberg), individual CBT (Heimberg), standard CT (Clark and Wells), CT (Clark and Wells) with shortened sessions, exposure in vivo, mindfulness training, IPT, psychodynamic psychotherapy, self-help (book) with and without support, self-help (internet) with and without support, and supportive therapy.
The model also considered treatment with pill placebo, consisting, in terms of resource use, of GP visits only, as well as waitlist as alternative treatment options, in order to assess the cost effectiveness of active interventions versus a non-specific medical management (represented by pill placebo) and a ‘do nothing’ option (represented by waitlist). Combined interventions (comprising concurrent provision of both pharmacological and psychological interventions) were not considered in the model structure because the GDG judged that the respective evidence was very limited (each combination therapy included in the guideline systematic review was assessed in one single small trial). Moreover, in many trials combined interventions were found to be less effective than their components and were associated with increased side effects and lower tolerability, so they were obviously less cost effective (more intensive and less effective) than single interventions; consequently there was no need for a formal evaluation of their cost effectiveness.
Model structure
A hybrid decision-analytic model consisting of a decision-tree followed by a two-state Markov model was constructed using Microsoft Office Excel 2007. The model estimated the total costs and benefits associated with provision of various interventions to adults with social anxiety disorder. The structure of the model, which aimed to simulate course of illness and relevant clinical practice in the UK, was also driven by the availability of clinical data.
According to the model structure, hypothetical cohorts of adults with social anxiety disorder were initiated on each of the 28 treatment options assessed, including treatment with pill placebo or inclusion in a waitlist. The duration of initial treatment was 12 weeks for drugs and pill placebo; for psychological interventions it varied by intervention (range between 9 and 16 weeks). In order to estimate QALYs it was assumed that psychological interventions lasted 12 weeks as well, which was consistent with the trial data and with clinical practice. Following treatment, people in each cohort either recovered (that is, they did not meet criteria for a diagnosis of social anxiety disorder any longer) or did not recover. Those recovering were given 6 months of maintenance therapy if they had been initiated on pharmacological treatment in order to sustain the treatment effect. No booster sessions were modelled for psychological interventions, as clinical evidence indicated that these are not necessary for sustained treatment effect. People who did not recover were assumed to stop treatment rather than switch to an alternative intervention; according to the expert opinion of the GDG, because of the nature of the disorder people are usually reluctant to keep in contact with health services and try an alternative treatment option.
During the year post-treatment, people who had recovered might experience a relapse, meaning that they again met the criteria for social anxiety disorder. People who had not recovered following treatment were assumed to remain in a state of social anxiety and not to recover spontaneously over this year. From that point on, all people in each cohort – both those who no longer met criteria for social anxiety disorder (that is, those who recovered and did not relapse) and those who met criteria (that is, those who recovered but relapsed as well as those who did not recover following therapy) – were entered into the Markov model. They could remain in the same health state or move between the two states of ‘no social anxiety’ and ‘social anxiety’. The Markov model was run in yearly cycles. A half-cycle correction was applied. Because of lack of long-term comparative clinical data, transitions between the two health states in the Markov model were assumed to be independent of intervention received at the start of the model.
The analysis considered two time horizons in order to explore short and longer-term costs and benefits: (1) intervention time (12 weeks) plus 1 year post-treatment (represented by the decision tree); and (2) intervention time (12 weeks) plus 5 years post-treatment (consisting of the decision tree and four yearly cycles of the Markov model). The GDG was interested in the long-term cost, benefits and cost effectiveness of the interventions assessed in the analysis and focused on the 5-year post-treatment results. However, 1-year post-treatment findings were also reviewed in order to explore the changes in the relative cost effectiveness of interventions over time. A schematic diagram of the economic model structure is presented in Figure 7.

Figure 7
Schematic diagram of the economic model constructed for the assessment of the relative cost effectiveness of interventions for adults with social anxiety disorder.
Costs and outcomes considered in the analysis
The economic analysis adopted the perspective of the NHS and PSS, as recommended by NICE (2009b). Costs consisted of intervention costs (healthcare professional time, drug acquisition and equipment/infrastructure required for self-help interventions) and other health and social care costs incurred by people with social anxiety disorder not recovering following treatment or experiencing a relapse following recovery (including GP consultations, home visits from health and social services, counselling or therapy contacts, and inpatient and outpatient secondary care). A secondary analysis that adopted a wider perspective which, in addition to NHS and PSS costs, considered receipt of social security benefits by people with social anxiety disorder was also undertaken. The measure of outcome was the QALY.
Clinical input parameters and overview of methods employed for evidence synthesis
Clinical model input parameters consisted of the probability of recovery at end of treatment, the probability of relapse following recovery within the first year post-treatment, as well as the probabilities of recovery and relapse in the 4-year Markov model phase.
The probability of recovery at end of treatment for all interventions was derived from the NMA undertaken for this guideline. The clinical effectiveness of all interventions was expressed in the form of SMDs. For the economic analysis, the SMDs were transformed into LORs as described in Chapter 3, and these were further transformed into probabilities of recovery, using as baseline the absolute probability of recovery for waitlist, which was estimated from available recovery data on waitlist arms in RCTs that were included in the guideline systematic review. The 40,000 iterations that were recorded in WinBUGS (as described in Chapter 3) were thinned by 4 so as to obtain 10,000 iterations for use in the economic model. This transformation of SMDs derived from the NMA into LORs and the subsequent indirect estimation of probability of recovery for every intervention assessed in the economic analysis was necessary for three reasons:
- The recovery data reported in the RCTs included in the guideline systematic review were sparse and not available for all interventions assessed in the economic analysis: of the 101 studies included in the NMA, only 25 reported recovery data; such data were available for 14 out of the 29 interventions considered in the economic analysis. Consequently, available recovery data were not adequate for populating all arms of the economic model.
- The economic analysis needed to reflect (and thus utilise) the same relative treatment effects that were estimated in the NMA, which determined the comparative clinical effectiveness of the interventions considered in this guideline.
- The methodology adopted allowed estimation of probability of recovery for every intervention included in the economic analysis while preserving the effect of randomisation, because the probability of recovery of each intervention was ‘linked’ to the relative treatment effect of the intervention as estimated in the NMA.
The results of the NMA that were used to populate the economic model are provided in Table 15. The table shows the probability of recovery at end of treatment for each intervention considered in the economic analysis. Treatment options have been ranked from most to least efficacious in terms of mean probability of recovery.
Table 15
Results of the NMA that were utilised in the economic model – probability of recovery at end of treatment.
The probability of relapse after recovery within the first year following pharmacological intervention was estimated based on relevant data reported in relapse prevention studies included in the guideline systematic review. Five placebo-controlled trials assessed the efficacy of pharmacological interventions in preventing relapse in people with social anxiety disorder: four of them assessed an SSRI (KUMAR1999 and STEIN2002b assessed paroxetine, MONTGOMERY2005 escitalopram, VAN-AMERINGEN2001 sertraline) and one assessed pregabalin (GREIST2011). All five studies reported a 6-month ‘drug’ relapse rate for people with social anxiety disorder who had responded to initial drug treatment (12 weeks) and were maintained on drug treatment during the 6 months of the trial (therefore the 6-month ‘drug’ relapse rate referred to participants who relapsed while taking an active drug as maintenance treatment), as well as a 6-month ‘placebo’ relapse rate for people with social anxiety disorder who had responded to initial 12-week drug treatment and received placebo during the 6 months of the study (therefore the 6-month ‘placebo’ relapse rate referred to participants who had responded to 12-weeks of initial drug treatment but then were discontinued from the drug and were given placebo instead). The economic model structure assumed that within the first year following initial drug treatment people who recovered received 6 months of maintenance treatment. Assuming that drug maintenance treatment does provide a benefit and reduces the risk of relapse (compared with discontinuation of the drugs immediately after the initial 12-week treatment), the risk of relapse in the 6 months following maintenance treatment should be lower than the pooled ‘placebo’ relapse rate from the placebo arms of the relapse prevention studies. Conversely, the risk of relapse after stopping the 6-month maintenance treatment should be higher than the pooled ‘drug’ relapse rate from the active drug arms of the relapse prevention studies, which was recorded while people were still on a drug. It was therefore assumed that over the first year following pharmacological intervention people who recovered were maintained on their initiated drug for 6 months and experienced relapses at the ‘drug’ relapse rate, and, after stopping the drug, for the remaining 6 months, they continued to experience some relapses at an overall (annual) rate that was lower than the 6-month ‘placebo’ relapse rate (it was assumed that the ‘placebo’ relapse rate did not increase after 6 months following discontinuation, and therefore the annual ‘placebo’ relapse rate should not be different from the 6-month ‘placebo’ relapse rate). For the purposes of simplicity and because of a lack of more suitable data, it was assumed that the probability of relapse after recovery within the first year following pharmacological treatment equalled the midpoint between the pooled ‘drug’ relapse rate and the pooled ‘placebo’ relapse rate reported in the relapse prevention studies included in the guideline systematic review. This estimate was utilised in all decision nodes of the model that involved drug treatment because relapse data for drugs were sparse and not available for the majority of pharmacological interventions considered in the economic analysis.
The probability of relapse following recovery in the pill placebo arm of the model was assumed to equal that for drug arms. This probability was deliberately not set to equal the ‘placebo’ relapse rate because people who had recovered in this arm had not been initiated on a drug (so they did not experience drug discontinuation that might potentially lead to an increase in the risk of relapse similar to the relapse rate of the placebo arms of the relapse prevention studies).
The probability of relapse after recovery within the first year following psychological intervention was calculated using the respective probability of relapse for drugs, estimated as described above, and the risk ratio (RR) of relapse between drugs and psychological interventions. The latter was derived from an observational study that evaluated the effects of maintenance treatment with phenelzine and group CBT (Liebowitz et al., 1999). The study followed an RCT that compared phenelzine, group CBT, pill placebo and psychological placebo (HEIMBERG1998). People who were initiated on either phenelzine or group CBT and had responded to 12 weeks of treatment (N = 28) were continued on their initial treatment for 6 months, and followed up for another 6 months during which they received no treatment. The study reported relapse rates over the 6-month maintenance treatment period, the 6-month follow-up period, and the combined 12-month period. A risk ratio of relapse for drugs (represented by phenelzine) versus psychological intervention (represented by group CBT) was estimated using the 12-month combined relapse data reported in the study. The probability of relapse after recovery for the psychological arms of the model was subsequently calculated as:
This probability was applied to all psychological intervention arms of the economic model, since no differential relapse data for the range of psychological interventions considered in the model are available in the literature.
The probability of relapse after recovery in the waitlist arm of the model was based on data reported in a prospective naturalistic study that followed people with anxiety disorders over 12 years (Bruce et al., 2005). The study followed 176 people with social anxiety disorder and reported a 12-year probability of recurrence, calculated using standard survival analysis methods. This probability allowed estimation of an annual probability of relapse that was applied to the first year after recovery in the waitlist arm.
The annual probabilities of recovery and relapse for all treatments in the 4-year Markov model phase were assumed to be independent of initial treatment and were also based on data reported in Bruce and colleagues (Bruce et al., 2005). In addition to the 12-year probability of recurrence, the authors also calculated a 12-year probability of recovery using survival analysis, which was used to estimate an annual probability of recovery. The estimated annual probabilities of recovery and relapse were applied to all cohorts in the economic model, regardless of initial treatment, in years 2 to 5 post-treatment (that is, in the Markov phase of the model).
Utility data and estimation of quality-adjusted life years
In order to express outcomes in the form of QALYs, the health states of the economic model needed to be linked to appropriate utility scores. Utility scores represent the Health Related Quality of Life (HRQoL) associated with specific health states on a scale from 0 (death) to 1 (perfect health); they are estimated using preference-based measures that capture people's preferences on the HRQoL experienced in the health states under consideration.
The systematic search of the literature identified one study that reported utility scores for specific health states associated with social anxiety (François et al., 2008) and two studies that reported utility data for adults with social anxiety disorder (and adults without a mental disorder), without differentiating between distinct health states of the condition: (1) Alonso and colleagues (2004), with data analysed and reported in Kaltenthaler and colleagues (2006), and (2) Saarni and colleagues (2007).
François and colleagues (2008) generated utility scores using SF-36 data derived from 517 people with social anxiety disorder who participated in 12 weeks of open-label treatment with escitalopram. Those responding to treatment were entered into a double-blind, placebo-controlled, multinational clinical trial of escitalopram for relapse prevention (MONTGOMERY2005). Participants were included in the open-label phase if they had had a primary diagnosis of generalised social anxiety and a score of 70 or more on the LSAS. Response to treatment was defined as a Clinical Global Impression-Improvement (CGI-I) score of 1 or 2; relapse was defined as either an increase in LSAS total score of 10 or more points or withdrawal of the participant from the study because of lack of efficacy as judged by the investigator. SF-36 data were obtained from participants at baseline, the end of the open-label period, and at 12 and 24 weeks after randomisation. Participants who did not complete the study attended an early discontinuation visit, at which the SF-36 was administered. SF-36 scores were converted into utility scores using the SF-6D algorithm (Brazier et al., 2002). The SF-6D algorithm has been generated using the standard gamble (SG) technique in a representative sample of the UK general population.
Alonso and colleagues (2004) reported EQ-5D and SF-36 data of people participating in a large, community-based mental health European survey, the European Study of the Epidemiology of Mental Disorders. Participants were members of the general population that underwent psychiatric assessments and completed various HRQoL instruments. The authors conducted additional analyses to those reported in their publication and generated EQ-5D and SF-36 utility scores for people who had experienced a wide range of mental disorders over the past 12 months (among whom 219 had social anxiety) and 19,334 people without a mental disorder over the past 12 months. Estimated utility scores were subsequently provided to the research team that conducted the economic analysis for the NICE TA on the use of CCBT for depression and anxiety (Kaltenthaler et al., 2006). Thus, EQ-5D and SF-6D utility scores derived from the European Study of the Epidemiology of Mental Disorders are available in Kaltenthaler and colleagues (2006). Utility scores for EQ-5D have been elicited from the UK general population using the time trade-off technique (TTO) (Dolan et al., 1996; Dolan, 1997). The SF-6D algorithm has been generated using SG in a representative sample of the UK general population (Brazier et al., 2002).
Saarni and colleagues (2007) reported EQ-5D data obtained from people aged 30 years or older, participating in a national health survey in Finland. The survey consisted of a health interview, a health examination and self-report questionnaires. The study reported EQ-5D utility scores for people who had experienced a range of mental disorders over the past 12 months (among whom 60 had social anxiety anxiety, with 14 having pure social anxiety) and 5,279 people with no mental disorder over the last 12 months. The authors used the UK TTO tariff (Dolan, 1997) in order to estimate utility scores from EQ-5D data.
Table 16 summarises the methods used to derive and value health states associated with social anxiety disorder in the literature and presents the respective utility scores reported in the three utility studies that were identified by the systematic search of the literature.
Table 16
Summary of studies reporting utility scores for social anxiety.
According to NICE guidance (NICE, 2013) on the selection of utility values for use in cost-utility analysis, the measurement of changes in HRQoL should be reported directly from people with the condition examined, and the valuation of health states should be based on public preferences elicited using a choice-based method, such as the TTO or SG, in a representative sample of the UK population. NICE recommends the EQ-5D (Brooks, 1996; Dolan, 1997) as the preferred measure of HRQoL in adults for use in cost-utility analysis. When EQ-5D scores are not available or are inappropriate for the condition or effects of treatment, NICE recommends that the valuation methods be fully described and comparable to those used for the EQ-5D (NICE, 2013).
The study by François and colleagues (2008) was the only one that reported utility data for different health states of social anxiety disorder. However, the GDG questioned the quality of the data because of the methodological limitations of MONTGOMERY2005, such as the high attrition rates. Moreover, the GDG felt that the utility data reported in the study represented a rather narrow benefit in HRQoL, as the difference in the utility scores between the states of response and non-response was only 0.031; for comparison, a study with similar design that estimated utility scores in responders and non-responders in generalised anxiety reported a respective difference of 0.13 (Allgulander et al., 2007). In addition, the reduction in utility for those relapsing following response was 0.017 in people with social anxiety disorder according to François and colleagues, and 0.03 in people with generalised anxiety according to Allgulander and colleagues. The GDG therefore judged that utility data reported by François and colleagues might have failed to capture the true benefit in HRQoL once a person with social anxiety disorder responds to treatment, and the true loss in HRQoL once the person relapses following response.
It should be noted that François and colleagues compared their findings with those of Allgulander and colleagues and admitted that ‘the effect of escitalopram on HRQoL was somewhat more modest in patients with generalised social anxiety disorder than in those with generalised anxiety disorder’. However, it was not the effect of escitalopram that was responsible for the discrepancies in the utility changes between the two studies and populations because utility changes reflected alterations in HRQoL once a person had/had not experienced response or relapse, with the two states being defined in a similar way in the two studies. Another point for consideration was that the GDG was interested in the utility of the recovery state, whereas the data reported in François and colleagues referred to the state of response. Finally, François and colleagues reported utility values based on the SF-6D, which is not the NICE preferred measure for use in cost-utility analysis. For all the above reasons the GDG decided not to use the utility data reported in François and colleagues, despite these being the only utility data capturing HRQoL in different health states of social anxiety disorder that were identified in the literature.
The GDG then assessed the EQ-5D-based utility data reported in Alonso and colleagues (2004) and the utility data from Saarni and colleagues (2007). The two studies were very similar in terms of design and reported utility data for people with social anxiety disorder over the last 12 months and for people without a mental disorder over the last 12 months. It was agreed that the utility data for the former could be used for the state of non-recovery or relapse (‘social anxiety’) in the guideline economic model; the utility data for people without a mental disorder over the last 12 months could be used as a proxy for the state of recovery (‘no social anxiety’). It was acknowledged that this is probably not a very accurate proxy because people recovering from social anxiety disorder may not reach the HRQoL of a person without a mental disorder over the last 12 months. Another limitation of these data is that the diagnosis of social anxiety disorder referred to a period of 12 months prior to the study, so some participants in both studies might have experienced an improvement in their condition over this period (and actually might not have social anxiety disorder at the point of interview). Nevertheless, the GDG accepted these as reasonable limitations and decided to use the data by Saarni and colleagues (2007) in the base-case analysis (which reflect a greater improvement in HRQoL following recovery), and to use the more conservative data by Alonso and colleagues in sensitivity analysis. Utility data from both studies are based on the EQ-5D UK tariff and therefore are in accordance with NICE guidance on the selection of utility data for use in cost-utility analysis (NICE, 2013).
It was assumed that the improvement in utility for people with social anxiety disorder recovering following treatment occurred linearly over the duration of treatment, starting from the utility value of social anxiety disorder and reaching the utility value of no social anxiety disorder. The duration of all treatments considered in the analysis was assumed to be 12 weeks in order to simplify calculation of utilities in people improving following treatment across cohorts. All changes in utility between the two states of ‘social anxiety’ and ‘no social anxiety’ were assumed to occur linearly over the time period of the change.
Side effects from medication are expected to result in a reduction in utility scores of people with social anxiety disorder. Disutility because of side effects was not considered in the analysis because the model structure did not incorporate side effects. This was due to inconsistent reporting of specific side effect rates across the studies included in the guideline systematic review. Moreover, no studies on people with social anxiety disorder reporting ‘disutility’ because of side effects were identified in the literature. However, Revicki and Wood (1998) examined the effect of side effects from antidepressants in the HRQoL of people with depression. According to the study, people with a side effect reported lower utility scores compared with those not experiencing side effects. The observed mean disutility ranged from 0.01 for dry mouth and nausea to 0.12 for nervousness and light-headedness. However, except for light-headedness and dizziness, the reduction in utility caused by side effects did not reach statistical significance. The GDG felt that it may be reasonable to extrapolate this evidence to the population of people with social anxiety disorder; consequently, it is possible that lack of consideration of disutility because of side effects has not had a great impact on the results of the economic analysis. Nevertheless, omission of the negative impact of drugs on HRQoL of adults with social anxiety disorder is acknowledged as a limitation of the analysis because it may have resulted in an over-estimation of the cost effectiveness of pharmacological interventions relative to psychological interventions considered in the model.
Cost data
Costs considered in the economic model consisted of intervention costs and extra health and social care costs incurred by adults with social anxiety disorder not recovering following treatment or relapsing following recovery. In addition, a secondary analysis considered receipt of social security benefits by adults with social anxiety disorder not recovering or relapsing following recovery.
Pharmacological intervention costs consisted of drug acquisition costs and GP visit costs. Intervention costs of placebo related to GP visit costs only. Costs were calculated by combining resource use estimates with respective national unit costs. Drug acquisition costs were taken from the NHS Electronic Drug Tariff, February 2013 (NHS Business Services Authority Prescription Pricing Division, 2013). For each drug the lowest reported price was selected and used in the analysis; where available, costs of generic forms were considered. The average daily dosage of each drug was determined according to optimal clinical practice (according to GDG expert opinion) and was consistent with the respective average daily dosage reported in the RCTs considered in the NMA that informed the economic model. Initial treatment with drugs was estimated to last 12 weeks, while people recovering following drug treatment received another 6 months (26 weeks) of maintenance treatment at the same daily dosage. All people under any pharmacological treatment (or placebo) were assumed to visit their GP four times over the 12 weeks of initial treatment; in addition, those recovering were assumed to pay three extra GP visits during maintenance treatment. The GP unit cost (£43 per patient contact lasting 11.7 minutes) was taken from Unit Costs of Health and Social Care 2012 (Curtis, 2012). This figure includes direct care staff costs and qualification costs.
Details on the resource use and total intervention costs of pharmacological interventions for adults with social anxiety are presented in Table 17.
Table 17
Average daily dosage, drug acquisition costs and total intervention costs of pharmacological interventions for adults with social anxiety disorder included in the economic model (2012 prices).
Intervention costs of psychological interventions were also calculated by combining resource use estimates with relevant national unit costs. Resource use estimates in terms of therapists' time were based on relevant data reported in RCTs included in the NMA that informed the economic model. For self-help studies the additional cost of a book or a computerised program was considered. All psychological interventions were assumed to be delivered by Band 7 clinical psychologists because this is broadly consistent with the type of therapists who delivered the interventions in the majority of RCTs included in the NMA. The unit cost of a Band 7 clinical psychologist per hour of client contact has been estimated based on the median full-time equivalent basic salary for Agenda for Change Band 7 and includes salary, salary on-costs and overheads, but excludes qualification costs because the latter are not available for clinical psychologists (Curtis, 2010). However, exclusion of qualification costs from the clinical psychologist unit cost would underestimate the total psychological intervention costs and would therefore, in all likelihood, overestimate their cost effectiveness relative to pharmacological interventions. In order to consider the qualification cost for clinical psychologists, a number of mental health professionals with different qualifications and salary bands were selected (for example, consultant psychiatrists and mental health nurses) and the reported unit costs for these professions with and without qualification costs were compared. The rate of unit costs without/with qualification costs was found to be 0.85, and this allowed estimation of a unit cost for Band 7 clinical psychologists at £101 per hour of client contact in 2012 prices, which included qualification costs. This cost was used in the base-case analysis. A one-way sensitivity analysis tested delivery of a self-help intervention by a Band 5 therapist (such as a mental health nurse) and delivery of group therapies by one Band 7 and one Band 6 (for example, trainee in clinical psychology) therapist.
In addition to therapists' time, the intervention costs of all psychological interventions included an initial GP visit for referral to psychological services. Moreover, the intervention costs of self-help programmes included the cost of either a book or a computerised program and related infrastructure/equipment required for the delivery of such a program (licence fee or website hosting, personal computers [PCs] and capital overheads).
The cost of a book for self-help was based on the cost of Rapee's Overcoming Shyness and Social Phobia: A Step by Step Guide (Rapee, 1998) available in the market (£22.95). The website hosting cost of computerised self-help was estimated based on information provided by the GDG, relating to a pilot research internet-based self-help program for people with social anxiety disorder currently tested in England. According to this information, the annual cost of secure internet hosting reached £14,000 (including maintenance and software bug fixing of the program), and was paid at an individual service level. Based on IAPT audit of activity data (information provided by the GDG), an average IAPT service sees about 2,500 people every year, of which 1.5% are estimated to have social anxiety disorder. Assuming 80% of these are offered (and accept) internet-based self-help, this means 30 people with social anxiety disorder use the internet-based self-help program, resulting in a website hosting cost of £467 per person. Since the particular internet-based self-help program was developed for research purposes, no licence fee was considered at the estimation of the intervention cost, although this cost component, which may be considerable, needs to be taken into account in the assessment of cost effectiveness of other computerised self-help packages for social anxiety disorder that may be available in the future. The annual costs of hardware and capital overheads (space around the PC) were based on reported estimates made for the economic analysis undertaken to inform the NICE TA on CCBT for depression and anxiety (Kaltenthaler et al., 2006) and equal £161 and £1,070, respectively (in 2012 prices). Kaltenthaler and colleagues (2006) estimated that one PC can serve around 100 people with a mental disorder treated with computerised programs per year. Assuming that a PC is used to full capacity (that is, it serves no fewer than 100 people annually, considering that it is available for use not only by people with social anxiety disorder, but also by people with other mental disorders, such as depression), the annual cost of hardware and capital overheads was divided by 100 users, leading to a hardware and capital overheads cost per user of £13. It must be noted that if users of such programs can access them from home or a public library, then the cost of hardware and capital overheads to the NHS is zero.
No booster (maintenance) sessions were assumed for psychological interventions. The intervention cost of waitlist was zero. Table 18 presents the resource use elements and the estimated intervention costs of all psychological interventions considered in the model.
Table 18
Resource use and estimated intervention costs of psychological interventions (2012 prices).
Costs of treating side effects of drugs were not considered in the economic analysis due to lack of consistency in reporting appropriate side effect data across all drugs. Nevertheless, the GDG estimated that the majority of common side effects, such as nausea, insomnia, sexual problems, dizziness, fatigue, palpitations and tachycardia, would be discussed during GP monitoring, which was considered at the estimation of intervention costs relating to initial and maintenance pharmacological intervention. Regarding less common side effects, such as hypertension (associated with SNRIs) and gastrointestinal bleeding (associated with SSRIs), these were thought to result in higher management costs at an individual level, but given their low frequency they were deemed to entail smaller economic implications at a study population level. Therefore, although the omission of costs associated with management of side effects is acknowledged as a limitation of the analysis, it is not considered to have substantially affected the economic modelling results.
The extra health and social care costs incurred by adults with social anxiety disorder not recovering post-treatment or relapsing following recovery were taken from Patel and colleagues (2002). The authors analysed service use data on 63 people with social anxiety and 8,501 people without psychiatric morbidity derived from the Psychiatric Morbidity Survey conducted in the UK in 1993–1994 (Meltzer et al., 1995). The study combined data on GP consultations, home visits from health and social services, counselling or therapy contacts and inpatient and outpatient secondary care with relevant national unit costs and subsequently estimated an annual total health and social care cost incurred by people with social anxiety disorder and people without psychiatric morbidity. People with social anxiety disorder in the model were estimated to incur the annual total health and social care cost for this population reported in Patel and colleagues, whereas people who recovered and were in the state of ‘no social anxiety’ were assumed to incur the respective cost incurred by people without psychiatric comorbidity reported in the study. People who relapsed following recovery during the first year post-treatment were assumed to incur the ‘social anxiety’ health and social care cost for 6 months and the ‘no social anxiety’ health and social care cost for the remaining 6 months.
Patel and colleagues also reported the mean annual value of social security benefits for people with social anxiety disorder and those without psychiatric comorbidity, and these costs were used in a secondary analysis that adopted a wider perspective in order to capture the broader economic implications of social anxiety disorder.
Health and social care costs as well as social security benefits were assumed to be the same across all arms of the economic model during the period of initial (12-week) treatment and therefore were excluded from further consideration.
All costs were expressed in 2012 prices, uplifted, where necessary, using the Hospital and Community Health Services Pay and Prices Index (Curtis, 2012). Costs and QALYs were discounted at an annual rate of 3.5%, according to NICE guidance (NICE, 2009b).
Table 19 reports the values of all input parameters utilised in the economic model and provides information on the distributions assigned to specific parameters in probabilistic analysis, as described in the next section.
Table 19
Input parameters utilised in the economic model of interventions for adults with social anxiety disorder.
Handling uncertainty
Model input parameters were synthesised in a probabilistic analysis. This means that all model input parameters were assigned probability distributions (rather than being expressed as point estimates), to reflect the uncertainty characterising the available clinical and cost data. Subsequently, 10,000 iterations were performed, each drawing random values out of the distributions fitted onto the model input parameters. Results (mean costs and QALYs for each intervention) were averaged across the 10,000 iterations. This exercise provided more accurate estimates than those derived from a deterministic analysis (which utilises the mean value of each input parameter ignoring any uncertainty around the mean), by capturing the non-linearity characterising the economic model structure (Briggs et al., 2006).
The distributions of the probability of recovery following treatment (year 1 of the model), which were obtained from the NMA, were defined directly from values recorded in each of the 10,000 respective iterations performed in WinBUGS and used in the economic analysis, as described earlier. The log-odds of recovery on waitlist was assumed to follow a normal distribution with mean −2.629 and variance 1.235. The LORs of recovery for each treatment relative to waitlist, as estimated by the WinBUGS model (described in Chapter 3), were applied to simulated values of this normal distribution and converted onto the probability scale. This ensured that the full posterior distribution of the relative treatment effects was used to estimate the absolute probabilities of recovery for each treatment.
The distribution of the probability of relapse for drugs was determined by assigning beta distributions to the pooled relapse rates reported for drug arms and placebo arms in the four relapse prevention RCTs included in the guideline systematic review. The risk ratio of relapse of drugs versus psychological interventions was assigned a log-normal distribution. Utility values were assigned beta distributions using the method of moments. The distributions of the annual probabilities of recovery and relapse in years 2–5 of the model were determined by assigning beta distributions to the 12-year respective probabilities that were used to estimate annual probabilities. The estimation of distribution ranges was based on available data in the guideline meta-analysis and the published sources of evidence.
Uncertainty in intervention costs was taken into account by assigning different probabilities in the number of GP visits (pharmacological interventions) or number of sessions (individual psychological interventions) attended by adults with social anxiety disorder. These probabilities were determined by data reported in the respective RCTs included in the NMA, such as completion rates, average number of sessions attended, and so on. Regarding pharmacological interventions, the same completion rate was applied to all drugs due to lack of relevant data specific to each of the drugs considered in the model. Based on data reported in large pharmacological intervention RCTs included in the NMA (N > 100), the completion rate of the 12-week initial treatment with pharmacological interventions was estimated at 75%. It was therefore assumed that 65% of people in each drug arm of the model attended four GP visits (as described in Table 17) and 10% attended either one less or one or two more visits (which might be occasionally required for the management of side effects). The 25% of people discontinuing the 12-week drug treatment were assumed to pay one or two visits to their GP. People discontinuing treatment were assumed to incur only 50% of the 12-week drug acquisition cost; in addition, if they recovered, they were assumed not to continue with the 26-week maintenance treatment. People who recovered and were thus offered 26 weeks of maintenance treatment were assumed to attend three GP visits (as described in Table 17) at a probability of 55%. The remaining 45% were assumed to pay either fewer visits (0 to 2) or one more visit because of side effects. If the number of GP visits during maintenance treatment equalled zero, no 26-week drug acquisition costs were considered in the model.
Regarding individual psychological interventions, based on relevant reported data, the completion rate was estimated at approximately 85% for all interventions except standard CT (Clark and Wells), which reached a 100% completion rate in the respective RCTs. According to the studies, participants were broadly considered as completers if they had missed no more than four sessions in total. Using this information and the average number of sessions in each arm of a trial or in the subgroup of completers, where reported, the following assumptions were made for all individual psychological interventions with the exception of standard CT (Clark and Wells): 70% of people in each individual psychological intervention arm of the model attended the optimal number of sessions (as described in Table 18); another 15% of people completed treatment but attended one to four fewer sessions; the remaining 15% of people in each cohort discontinued treatment and attended randomly a lower number of sessions (missed five or more sessions and at minimum attended only one session of the intervention).
The cost of group psychological intervention was deemed to be stable and not subject to uncertainty, irrespective of compliance with treatment; this is because participants in a group are not replaced by another person when they occasionally miss one or more sessions or discontinue treatment. Therefore the same resources (in terms of healthcare professional time) are consumed and the full cost of treatment is incurred whether people attend the full course or fewer group sessions. Drug acquisition costs are also not subject to uncertainty. Consequently intervention costs of group psychological interventions and drug acquisition costs were not assigned probabilistic distributions. Extra health and social care costs for people not recovering or relapsing following recovery, as well as social security benefit costs, were assigned a gamma distribution, determined by data reported in the source study.
Table 19 provides details on the types of distributions assigned to each input parameter and the methods employed to define their range.
Extra probabilistic sensitivity analyses were also undertaken to explore the impact on the results of the following alternative scenarios:
- Adoption of a wider perspective which, in addition to NHS and PSS costs, considered receipt of social security benefits by people with social anxiety disorder, as reported in Patel and colleagues (2002).
- A change in the healthcare professional unit cost for self-help and group-based interventions: this scenario assumed delivery of self-help interventions by a Band 5 therapist (for example, a mental health nurse) and delivery of group interventions by one Band 7 and one Band 6 therapist (the latter reflecting the salary of a trainee in clinical psychology). The unit cost of a Band 5 mental health nurse was taken from Unit Costs of Health and Social Care 2012 (Curtis, 2012). The unit cost of a Band 6 trainee therapist was not available and was therefore assumed to be in the middle between the unit cost of a Band 5 mental health nurse and a Band 7 clinical psychologist.
- Use of utility data from Alonso and colleagues (2004) instead of Saarni and colleagues (2007).
Presentation of the results
Results of the economic analysis are presented as follows:
For each intervention, mean total costs and QALYs are presented, averaged across 10,000 iterations of the model. An incremental analysis is provided, where all options have been ranked from the most to the least effective (in terms of QALYs gained). Options that are dominated by absolute dominance (that is, they are less effective and more costly than one or more other options) or by extended dominance (that is, they are less effective and more costly than a linear combination of two alternative options) are excluded from further analysis. Subsequently, ICERs are calculated for all pairs of consecutive options remaining in the analysis.
ICERs are calculated by the following formula:
where ΔC is the difference in total costs between two interventions and ΔE the difference in their effectiveness (QALYs). ICERs express the extra cost per extra unit of benefit (QALY in this analysis) associated with one treatment option relative to its comparator. The treatment option with the highest ICER below the NICE lower cost-effectiveness threshold of £20,000 per QALY (NICE, 2008) is the most cost-effective option.
In addition to ICERs, the mean net monetary benefit (NMB) of each intervention is presented. This is defined by the following formula:
where E is the effectiveness (number of QALYs) and C the costs associated with the treatment option, and λ is the level of the willingness-to-pay per unit of effectiveness, set at the NICE lower cost-effectiveness threshold of £20,000 per QALY (NICE, 2008). The intervention with the highest NMB is the most cost-effective option (Fenwick et al., 2001). Moreover, for the most cost-effective intervention, the probability that it is the most cost-effective option is also provided, calculated as the proportion of iterations (out of the 10,000 iterations run) in which the intervention had the highest NMB among all interventions considered in the analysis.
Economic modelling results
The results of the economic analysis for the time horizon of 5 years post-treatment are provided in Table 20. This table provides mean QALYs and mean total costs for each intervention assessed in the economic analysis, as well as the results of incremental analysis, the NMB of each intervention, and its ranking by cost effectiveness (with higher NMBs indicating higher cost effectiveness). Interventions have been ordered from the most to the least effective in terms of number of QALYs gained.
Table 20
Results of economic modelling, 5 years post-treatment - base-case analysis: NHS and PSS perspective.
At 5 years post-treatment, standard CT (Clark and Wells) is the most effective intervention because it produces the highest number of QALYs. This result was not unexpected, given that the intervention had the highest probability of recovery among all interventions in the NMA. At the same time, it is the second most costly intervention, following psychodynamic psychotherapy. According to the NMBs provided in Table 20, standard CT (Clark and Wells) produces the highest NMB and therefore appears to be the most cost-effective intervention. Its ICER versus phenelzine (which is the next most effective intervention not dominated by absolute or extended dominance in incremental analysis) equals £8,426 per QALY, which is below the NICE lower cost-effectiveness threshold of £20,000 per QALY. The probability of standard CT (Clark and Wells) being the most cost-effective intervention is 69%, which reflects the proportion of the 10,000 iterations of the economic model in which it had the highest NMB among all interventions. According to the analysis, the second most cost-effective option at 5 years post-treatment is individual CBT. Phenelzine ranks third in terms of cost effectiveness, while book-based self-help without support ranks fourth. Individual CBT (Heimberg) ranks fifth and book-based self-help with support ranks sixth. Of the other individual psychological interventions, CT with shortened sessions ranks ninth, psychodynamic psychotherapy ranks 25th, and IPT ranks 26th, just above waitlist; supportive therapy is the least cost-effective intervention, ranking in 28th place. Group psychological interventions rank in places between 10 and 15, with the exception of mindfulness training, which ranks 23rd. Drugs (with the exception of phenelzine) rank between places 8 and 22, with paroxetine being the most cost-effective drug after phenelzine, followed by venlafaxine, fluvoxamine, sertraline, fluoxetine and escitalopram. Internet-based self-help ranks seventh (with support) and 20th (without support).
Figure 8 provides the cost effectiveness plane of the analysis, 5 years post-treatment. Each intervention is placed on the plane according to its incremental costs and QALYs compared with waitlist (which is placed at the origin).

Figure 8
Cost-effectiveness plane of all interventions for adults with social anxiety disorder assessed in the economic analysis plotted against waitlist - incremental costs and QALYs per 1000 adults with social anxiety disorder, 5 years after treatment.
Detailed results of the base-case economic analysis, with 95% CIs of costs and QALYs and disaggregation of costs are provided in Appendix 23.
Regarding 1 year post-treatment, phenelzine was the most cost-effective intervention among those considered in the analysis because it produced the highest NMB. Its ICER to paroxetine, which was the next most effective non-dominated intervention in incremental analysis, was £4,063 per QALY, while the ICER of standard CT (Clark and Wells) versus phenelzine exceeded £47,000 per QALY, which is well above the NICE cost-effectiveness threshold of £20,000 per QALY. The probability of phenelzine being the most cost-effective intervention at 1 year post-treatment was 55%. The second most cost-effective option at 1 year post-treatment was paroxetine, followed by book-based self-help (without support) and sertraline. Overall, results indicated that in the short term, drugs seemed to be more cost effective than psychological interventions for adults with social anxiety disorder: drugs ranked in the first 13 places, with the exception of places 3 (book-based self-help without support) and 11 (book-based self-help with support). The various forms of individual CBT, including CT (Clark and Wells), seemed to follow drugs and book-based self-help in terms of cost effectiveness. Group psychological interventions, internet-based self-help and other individual psychological interventions were less cost effective compared with drugs, book-based self-help, and individual forms of CBT. Results for 1 year post-treatment, including mean QALYs and costs with 95% CIs, disaggregation of costs, incremental analysis, NMBs, ranking of interventions by cost effectiveness and the cost-effectiveness plan are presented in Appendix 23.
Results were robust under all alternative scenarios examined in sensitivity analyses. Standard CT (Clark and Wells) was the most cost-effective intervention at 5 years post-treatment when a wider perspective that included social security benefits was adopted, alternative unit costs for self-help and group psychological interventions were assumed, and alternative utility values were used. Ranking of interventions in terms of cost effectiveness was broadly the same after using a wider perspective, and alternative unit costs and utility values. Results of secondary and sensitivity analyses can be found in Appendix 23. The economic evidence profile of the guideline economic analysis is provided in Appendix 24.
Discussion – limitations of the analysis
The guideline economic analysis assessed the cost effectiveness of a broad range of pharmacological and psychological interventions for adults with social anxiety disorder over 5 years post-treatment. In addition, 1-year post-treatment results were obtained and compared with the 5-year post-treatment results. This is because the GDG was interested in the potential changes in the relative cost effectiveness of interventions over time. The results of the analysis suggest that, although in the short-term pharmacological interventions appear to be, overall, more cost effective than psychological interventions, at 5 years post-treatment the relative cost effectiveness of individual forms of CBT improves significantly, so that standard CT (Clark and Wells), individual CBT, individual CBT (Heimberg) and CT (Clark and Wells) with shortened sessions rank first, second, fifth and ninth, respectively, in terms of cost effectiveness. The probability of standard CT (Clark and Wells) being the most cost-effective intervention at 5 years is 69%. Phenelzine is the third most cost-effective intervention. Book-based self-help also appears to be cost effective compared with other treatment options, with the two forms of it (with and without support) being among the six most cost-effective interventions of those assessed. Group-based psychological interventions do not appear to be particularly cost effective relative to other available treatments, ranking in places between 10 and 15, with the exception of mindfulness training, which ranks 23rd. Drugs (with the exception of phenelzine) are also not particularly cost effective, ranking between places 8 and 22; following phenelzine, the next most cost-effective drugs are (in order): paroxetine, venlafaxine, fluvoxamine, sertraline, fluoxetine and escitalopram. Internet-based self-help ranks seventh (with support) and 20th (without support). Other individual psychological interventions, such as psychodynamic psychotherapy, IPT and supportive therapy rank 25th, 26th and 28th, respectively.
The emergence of individual psychological interventions in the form of CBT as cost-effective options at 5 years can be attributed to two factors: first, over the 5-year time horizon there is a longer time period to accrue the benefits resulting from the differential relapse rate between psychological and pharmacological interventions, which was applied in the first year of the economic model. Based on the model input parameters, the proportion of people who relapse following post-treatment recovery is substantially lower if they receive a psychological, rather a pharmacological, intervention, and at 5 years post-treatment the benefit of being free from social anxiety has been enjoyed over a longer time period. Second, over a 5-year time horizon the high intervention costs of individual psychological interventions (which are responsible for the relatively low performance of these interventions in terms of cost effectiveness at 1 year post-treatment) are spread over a longer time period and are offset to a greater extent by NHS and PSS cost savings because there are fewer people relapsing and incurring such extra costs.
Results of the economic analysis were overall robust to different scenarios explored through sensitivity analysis. Results were practically unaffected when a wider perspective incorporating social security benefits was adopted, and when self-help and group psychological interventions were assumed to be delivered by less trained therapists. Moreover, using alternative utility data that assumed more conservative utility gains following recovery did not change the overall conclusions.
The clinical effectiveness data utilised in the model were derived from the NMA undertaken for this guideline. This methodology enabled evidence synthesis from both direct and indirect comparisons between interventions, and allowed simultaneous inference on all treatments examined in pairwise trial comparisons while respecting randomisation (Caldwell et al., 2005; Lu & Ades, 2004). The NMA utilised continuous data to estimate the relative treatment effects of interventions, and then transformed the estimated SMDs into probabilities of recovery, using waitlist as baseline, as discussed in Chapter 3. This was necessary in order to populate the economic model, as no comprehensive recovery data were available for the range of interventions assessed in the economic analysis. Moreover, the economic analysis needed to reflect the same relative treatment effects that were estimated in the NMA, which determined the comparative clinical efficacy of the interventions considered in this guideline. Transformation of SMDs into probabilities of recovery is valid as long as the relative treatment effect estimated using continuous data is equal to the treatment effect estimated using recovery data. Such an assumption cannot be checked for all interventions included in the economic analysis (since no recovery data are available for a large number of interventions); however, a comparison between continuous and recovery data indicated a strong relationship between them and therefore this transformation is unlikely to have introduced strong bias in the analysis (more details are provided in Chapter 3).
The assumptions and any limitations of the NMA model, as well as the limitations of individual studies considered in the NMA, have unavoidably impacted on the quality of the economic model clinical input parameters. For example, many of the included studies were not registered and both the clinical and economic results may be vulnerable to reporting and publication bias. The assumptions underlying the NMA model have been described in detail in Chapter 3; the characteristics and any limitations of the individual studies and the NMA model have been described in Section 6.5.2.
Treatment discontinuation because of side effects or other reasons was not considered in the model structure because no relevant data were systematically reported in the trials considered in the guideline systematic literature review. However, the probabilistic model did assume that a percentage of people might have not completed treatment or there might have been less than perfect compliance. In addition, most clinical efficacy data were analysed on an intention-to-treat basis and implicitly accounted for discontinuation.
One limitation of the model is the relapse data used to populate the model. Relapse data for pharmacological interventions are very sparse in the literature. Ideally, the economic model required drug-specific data on the probability of relapse after 6 months of maintenance treatment for adults with social anxiety disorder who have recovered following initial 12-week drug treatment. However, no such data were identified in the literature. Because of the lack of relevant relapse data specific to each drug considered in the analysis, the probability of relapse for all pharmacological interventions was assumed to be the same, and was estimated as the midpoint of the pooled relapse rates reported for drug and placebo arms in the relapse prevention RCTs included in the guideline systematic review. These rates referred to relapse during pharmacological maintenance treatment and relapse after discontinuation of initial (12-week) pharmacological treatment without maintenance, respectively. Moreover, relapse prevention studies measured relapse following response to treatment rather than recovery, which was the modelled outcome in the economic analysis. It is possible that the probability of relapse following recovery is lower than that following response to treatment and therefore the economic analysis may have potentially overestimated relapse following treatment. Furthermore, in reality, different drugs are likely to be associated with different risks for relapse, and this possibility has not been reflected in the economic model due to lack of drug-specific relapse data in the literature.
The RR of relapse of pharmacological versus psychological interventions was adopted from a small observational study (N = 28) that evaluated the effects of maintenance treatment with phenelzine and group CBT (Liebowitz et al., 1999), due to lack of other relevant data. Subsequently, as with pharmacological interventions, all psychological interventions were assumed to have the same risk of relapse due to lack of intervention-specific data, but, as in the case of drugs, this assumption may not hold. Nevertheless, the mean probabilities of relapse for pharmacological and psychological interventions estimated for the economic model (42% versus 14%, respectively) are very close to respective relapse rates reported for people with OCD (45% versus 12%, respectively) (Simpson & Fallon, 2000) and broadly consistent with respective figures reported for panic disorder (40% versus 5%, respectively) (Clark et al., 1994).
The RR of relapse of pharmacological versus psychological interventions was applied to the first year of the model only. For years 2 to 5 the model conservatively assumed that the same probability of relapse applied to all interventions, both psychological and pharmacological. This assumption may have favoured drugs, if the beneficial effect of psychological interventions relative to drugs in terms of reduced relapse rates, as indicated by Liebowitz and colleagues (1999), persists beyond 1 year.
Utility data used in the economic model were taken from a study that analysed survey data on people who had experienced social anxiety disorder (or other mental disorders) and people without a mental disorder over the 12 months prior to the survey interview. A limitation of these data is that the diagnosis of social anxiety disorder referred to a period of 12 months prior to the survey, so some participants might have experienced an improvement in their condition over this period, and might have actually recovered at the point of interview. Therefore, it is not certain that the HRQoL of this mixed group of people accurately reflects the HRQoL of the study population in the model (that is, people with a current diagnosis of social anxiety disorder). Moreover, the HRQoL of people without a mental disorder over the last 12 months may be higher than the HRQoL of people recovering from social anxiety disorder. However, after reviewing relevant literature, the GDG decided that these utility data were most appropriate to use in the economic model, because compared with other available utility data, they were judged to reflect more closely the HRQoL of adults with social anxiety disorder and those recovering following treatment, and also met the NICE criteria for the selection of utility data for cost-utility analysis.
Owing to the lack of comprehensive overall and specific side effect rates across all interventions, (dis)utility data due to side effects associated with drug treatment, and costs of treating these side effects, the model did not consider these parameters. Nevertheless, probabilistic analysis did take into account that a small proportion of people receiving pharmacological interventions may attend a higher number of GP visits for the management of side effects. In any case, omission of side effects from the model structure may have potentially led to overestimation of the cost effectiveness of drugs relative to psychological interventions, and may have had an impact on the relative cost effectiveness between different drugs.
Extra NHS and PSS costs incurred by people with social anxiety disorder not recovering or relapsing following recovery were taken from a study that utilised service use data from a national survey (Patel et al., 2002). The survey was conducted in 1993–1994 and is therefore outdated. However, it was not possible to identify recent data specific to UK service use of people with social anxiety disorder in the literature. The recent Adult Psychiatric Morbidity Survey (McManus et al., 2009b) did not report data specific to people with social anxiety disorder. More recent service use data for people with social anxiety disorder have been reported in a US study (Wang et al., 2005) and a study conducted in the Netherlands (Acarturk et al., 2008) but these refer to different healthcare settings and do not necessarily reflect UK relevant resource use. Therefore, the study by Patel and colleagues (2002) was the best source for obtaining this cost parameter for the economic model.
A secondary analysis that adopted a wider perspective incorporating social security benefits was undertaken. The relative cost effectiveness of interventions was practically unaffected by inclusion of such benefits. However, it must be noted that, because of a lack of more specific data, the model assumed that people recovering from social anxiety disorder received reduced benefits (equalling benefits received by people without a mental disorder), and then returned to receipt of higher social benefits (equalling benefits received by people with social anxiety disorder) if they relapsed. However, receipt of social benefits is a long-term process that is not necessarily directly related to events characterising the clinical course of social anxiety disorder, such as recovery or relapse, within a short period of time, such as the 5 years of the model time horizon. Thus this secondary analysis may have overestimated the reduction in social benefits received by people recovering following treatment.
Overall conclusions from the economic evidence
Existing economic evidence is very sparse in the area of interventions for adults with social anxiety disorder and is characterised by important limitations; therefore, it is difficult to draw conclusions on the cost effectiveness of interventions for adults with social anxiety disorder based on existing evidence.
The economic analysis undertaken for this guideline concluded that, although drugs appear to be, overall, more cost effective in the short-term, various forms of individual CBT such as standard CT (Clark and Wells), individual CBT and individual CBT (Heimberg) are overall more cost effective in the longer term. It is possible that the cost effectiveness of pharmacological interventions has been overestimated because the disutility associated with the presence of side effects from drugs was not taken into account in the analysis. Book-based self-help also appears to be cost effective compared with other treatment options; in contrast, group-based psychological interventions and other individual psychological interventions (such as psychodynamic psychotherapy, IPT and supportive therapy) appear to be less cost effective than individual forms of CBT, book-based self-help and pharmacological interventions. Supported internet-based self-help is a potentially cost-effective option, however it is not available in UK clinical practice yet, and the associated intervention costs used in the analysis were based on a relevant research programme currently being piloted in the UK. Once such an intervention becomes available in UK clinical practice, its cost effectiveness will need to be reassessed after taking into account relevant costs specific to the intervention (including any licence or internet hosting fees).
6.11. OVERALL CLINICAL SUMMARY
6.11.1. Pharmacological interventions
The review of clinical effects suggests that several pharmacological interventions may be efficacious in reducing symptoms of social anxiety disorder and may also improve mood, though the exclusion in some trials of participants with depression make it difficult to demonstrate this conclusively. The strongest evidence was for classes of drugs, which suggests that SSRIs, SNRIs, MAOIs and anticonvulsants may be efficacious. Main effects were large with overlapping CIs, all of which included the CIs for pill placebo; although there may be some differences in efficacy within classes, there was little evidence of this post-treatment. Among the SSRIs and SNRIs, escitalopram, fluvoxamine, fluoxetine, paroxetine, sertraline and venlafaxine were efficacious. The MAOIs phenelzine and moclobemide appear to be efficacious and there is some limited evidence for the anticonvulsants gabapentin and pregabalin. There was little evidence to support the use of other medications, including citalopram and levetiracetam. Among benzodiazepines, there was better evidence for clonazepam than for alprazolam. The health economic model identified phenelzine as the most cost-effective drug, although the GDG had concerns about the side effects (including hypotension), dietary restrictions, potential toxicity, and the quality of the data, which may have overestimated the effects; the GDG also notes that it is not licensed for social anxiety disorder. There was some evidence to support paroxetine, venlafaxine, fluvoxamine, sertraline, fluoxetine and escitalopram, if phenelzine were excluded from the analysis. The evidence reviewed also identified a number of other factors to consider in the use of those drugs thought to be efficacious, including: dietary restrictions associated with the use of MAOIs (in particular phenelzine); increased risk of blood pressure elevation and cardiac effects (for example, for venlafaxine) and hypotension (for example, for phenelzine); discontinuation symptoms with the antidepressants, particularly for paroxetine and venlafaxine; and tolerance and problems with withdrawal associated with the use of benzodiazepines.
In addition, the GDG reviewed existing NICE guidance (Depression [NCCMH, 2010; NICE, 2009a] and Generalised Anxiety Disorder and Panic Disorder [With or Without Agoraphobia] in Adults [NCCMH, 2011b; NICE, 2011c]) regarding the safe use of the drugs reviewed and the monitoring of side effects.
6.11.2. Psychological interventions
The strongest clinical evidence for large and sustained benefits supports the use of psychological interventions. This was particularly the case for CBT (individual and group), self-help (supported and unsupported), exposure and social skills, with more modest effects for short-term psychodynamic psychotherapy, IPT and mindfulness training, although for the latter two the effect was not significant.
Evidence suggests that psychological interventions also improve secondary outcomes, including depression and disability, and the benefits are sustained at follow-up.
Individual CBT had the largest clinical effect, and it was the only intervention in the NMA that was clearly superior to both waitlist and pill placebo. All manualised forms of individual CBT had very large effects; there was some evidence that the Clark and Wells model may be superior to other forms of CBT, but it should be noted that all trials were conducted by the developer. Manualised forms of group CBT also had large effects, particularly those following the Heimberg manual.
A number of interventions, including cognitive bias modification, exposure (which was efficacious as a stand-alone intervention but has been adapted into more recent and efficacious interventions) and social skills training, contained components of efficacious psychological interventions for social anxiety disorder. The GDG concluded that people with social anxiety disorder should be offered an integrated programme of treatment rather than separate components that, in the main, have not demonstrated clinical efficacy as stand-alone interventions. For example, exposure alone, although clinically efficacious, was found not to be cost effective.
The economic model identified individual standard CT (Clark and Wells) as the most cost-effective psychological intervention and the most cost-effective intervention overall, at 5 years after treatment. Over the same time horizon, individual CBT and individual CBT (Heimberg) were ranked as second and fifth most cost-effective interventions, respectively, whereas book-based self-help without and with support were ranked fourth and sixth most cost-effective interventions, respectively.
6.11.3. Interventions for fear of public speaking, sweating, and other subtypes
The evidence for the treatment of fear of public speaking (task concentration training and social skills) suggests that interventions that have been specifically developed for this fear were not effective in reducing symptoms of social anxiety disorder, but there was limited evidence for individual CBT. Psychological interventions focused specifically on blushing or sweating did not appear to be effective. In a study of inpatient settings no difference was identified between group CBT and group IPT.
The evidence does not suggest there are any benefits of botulinum toxin injections and thoracic sympathectomy on symptoms of social anxiety disorder. The GDG noted that both interventions may have a benefit for some physical symptoms in other populations (for example, people with hyperhidrosis), but there is no evidence of benefit for people with social anxiety disorder and the results of other trials are not applicable to this population.
There was no evidence to suggest that interventions that work for people with generalised social anxiety disorder would not work for people with the performance subtype or with specific primary fears.
6.11.4. Combined psychological and pharmacological interventions
Evidence for combined interventions, including for cognitive enhancers in addition to exposure, was of very low quality. No combination was tested in more than one trial, and the included trials included fewer than 200 participants having treatment. Estimated effects for some combinations were lower than the component therapies.
6.11.5. Interventions for comorbid disorders
There is only very low-quality evidence for the treatment of social anxiety disorder in trials that include only participants with a comorbid disorder including alcohol misuse (paroxetine) and ADHD (atomoxetine), which suggested no additional important benefit on symptoms of social anxiety disorder.
6.12. FROM EVIDENCE TO RECOMMENDATIONS
The GDG determined that the primary outcome was a clinically important reduction in symptoms of social anxiety disorder. The GDG would have preferred to have compared recovery (loss of diagnosis), but less than 25% of trials reported recovery and many trials reported only limited data beyond end-of-treatment scores. Symptoms at endpoint were therefore chosen as the main outcomes for use in the NMA. Effect sizes were adjusted using available recovery data and the clinical model was used to estimate recovery for a health economic model. The quality of the evidence was considered using the GRADE method for all pairwise comparisons; the quality of evidence analysed in the NMA was first examined through pairwise comparisons, then by considering quality (inconsistency, indirectness, imprecision, risk of bias and publication bias) for all interventions in the NMA. The economic model developed for this guideline assessed the cost effectiveness of pharmacological and psychological interventions over 1 and 5 years following treatment. Consideration of a 5-year time horizon was assessed as being the most important as this allowed assessment of the costs, effects and cost effectiveness of interventions in the longer term. The GDG therefore focused on the 5-year economic results in order to make recommendations on interventions for social anxiety disorder. However, long-term clinical data were limited and a number of assumptions were made in the economic analysis. Such assumptions are likely to have underestimated the long-term benefits of psychological interventions, as discussed in Section 6.7.
The clinical and economic analyses identified a number of potentially clinically and cost-effective interventions including individual CBT, CBT-based self-help, and medication including some SSRIs and MAOIs. In developing recommendations, the GDG was mindful of a number of important issues concerning the delivery of interventions for social anxiety disorder. In developing recommendations for pharmacological interventions the GDG took into account the following factors: the very limited long-term follow-up data with drugs and the attrition rates in some continuation studies, the side effects of the medication (for example, possible blood pressure changes with venlafaxine and phenelzine), discontinuation symptoms (with all SSRIs and paroxetine and venlafaxine in particular), potential drug interactions (with fluvoxamine), the small evidence base for fluoxetine relative to other drugs (N = 107), dietary restrictions with the MAOIs, the likelihood of relapse following discontinuation, and withdrawal and tolerance with the benzodiazepines. There was no evidence to support the use of beta-blockers for social anxiety disorder. In addition a number of the drugs that were identified as potentially clinically efficacious are rarely prescribed in primary care (where over 95% of prescriptions for social anxiety disorder are issued). These factors, along with a clear view from clinical and service user members of the GDG that most service users have a strong preference for psychological interventions, led the GDG to conclude that drugs should usually be a second-line treatment for social anxiety disorder. These factors, and the GDG's concerns about the relative seriousness and magnitude of risks of various side effects, also led to the development of a sequence of recommendations for the use of drugs in social anxiety disorder based on a balance of the benefits and disbenefits of treatment. SSRIs (escitalopram or sertraline) were recommended as first-line drug treatments, followed by fluvoxamine, paroxetine and venlafaxine, which although possibly as effective as the other SSRIs, were considered second-line pharmacological options because of concerns about side effects and discontinuation effects (with paroxetine and venlafaxine). The MAOIs were considered third-line pharmacological interventions because of the drug interactions, dietary restrictions and side effects.
The reviews undertaken for the Depression (NCCMH, 2010) and the Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults (NCCMH, 2011b) guidelines proved a strong evidence base on which the GDG could develop, through informal consensus methods and using their expert knowledge of social anxiety disorder, recommendations concerning the safe use of the drugs recommended in this chapter. Given the level of extrapolation from evidence on other disorders, the GDG was cautious in making recommendations but decided that in order to support the effective and safe delivery of pharmacological interventions specific advice was needed for people with social anxiety disorder. The GDG developed these recommendations in light of the recommendations on the clinical and cost-effectiveness of the pharmacological interventions (see Section 6.6).
With regard to specific recommendations the GDG felt it was important to inform service users of any possible side effects and what might be done to better manage them. The GDG was particularly concerned that the increased agitation sometimes seen with the use of SSRIs might present particular problems for people with social anxiety disorder if they were not informed of these risks before taking the drug. Although suicide risk is not as high in social anxiety disorder as in depression, because of the uncertainty about the risk of increased suicidality particularly in younger people the GDG felt it was important to draw prescribers' attention to these risks and ensure that adequate follow-up and monitoring is provided. Additional recommendations were also developed concerning pharmacological and dietary restrictions with the MAOIs, the management of short-term side effects and the requirement to gently taper most medication when stopping it.
The clinical and cost-effectiveness analyses established that individual CT (Clark and Wells model) was the most efficacious intervention but the GDG noted that the class effect for individual CBT was also very large and the different forms were largely overlapping in their likely effects. Even if the Clark and Wells model is excluded from consideration, individual CBT remains the most clinically and cost-effective intervention. The GDG considered a number of factors in developing the recommendation for individual CBT including the demands of training staff to deliver the intervention, the number and variety of trials (other than the model developers) supporting a model and the feasibility for use in the UK healthcare system. In light of this, the GDG decided to recommend two models of individual CBT, both of which are well-established: the Clark and Wells (Clark & Wells, 1995) and Heimberg (Hope et al., 2006) models. To guide practitioners in delivering these interventions, the GDG referred to the manuals used in clinical trials and extracted the key components of each therapy. The GDG was aware, however, that not all participants responded to individual CBT and wished to offer alternative psychological interventions (as is the case for the pharmacological interventions). The GDG did consider suggesting group CBT, but it is less efficacious than individual CBT and the economic model demonstrated that group CBT is also less cost effective than individual CBT. For people who do not want individual CBT, the GDG felt that a group form of the same treatment was not likely to be an acceptable option.
The GDG therefore decided to recommend two other psychological interventions as second-line psychological treatments. For people who do not want individual CBT, the GDG decided to recommend CBT-based supported self-help because the effects were greater than for unsupported self-help. Supported self-help offers a different mode of delivery from individual CBT and there was some evidence to suggest that it might be taken up by some people who would refuse an offer of face-to-face interventions (individual or group). In addition, self-help was identified as a cost-effective psychological intervention in the economic analysis. However, in making this recommendation the GDG was clear that supported self-help was not considered to be a ‘low-intensity intervention’ that could be offered to people with a milder form of social anxiety disorder or as a ‘stepped treatment’ to be offered before individual CBT. The GDG was also concerned to offer alternative treatments to individual CBT and CBT-based self-help because, in their expert opinion, people who wanted psychological treatment and had refused or not benefitted from individual CBT would be unlikely to take up or benefit from either group CBT or interventions such as social skills, exposure or cognitive bias modification, which share similar components to some CBT treatments. In developing a recommendation for alternative psychological treatments, the GDG wished to recommend treatments that had evidence of effect compared with waitlist and were established and used in the UK healthcare system. Using these criteria the GDG chose to recommend short-term psychodynamic psychotherapy specifically developed to treat social anxiety disorder (for which the evidence is weaker than for individual CBT and self-help), but with the important qualifier that before this intervention is considered the service user has to have been offered and declined CBT, supported self-help and pharmacological interventions. In this context, the GDG noted that although short-term psychodynamic psychotherapy has demonstrated effects compared with waitlist, the NMA suggests it is no more effective than psychological placebo.
The evidence for combination treatment was limited and of poor quality. However, the GDG, drawing on their expert opinion, did consider that the addition of an SSRI might facilitate the treatment of people receiving CBT who had not fully responded after a course of CBT, had made some progress and wished to continue with CBT.
The GDG was also concerned to limit the use of treatments for which it considered there to be insufficient evidence to support their use (that is, mindfulness training and supportive therapy), or where there was very limited evidence of benefit when the potential harms were considered (that is, TCAs, antipsychotics, anticonvulsants, beta-blockers and St John's wort). The GDG was also of the view that benzodiazepines had no role in the routine treatment of social anxiety disorder.
The use of physical interventions for perceived symptoms (for example, thoracic sympathectomy and botulinum toxin) are not recommended in the treatment of social anxiety disorder as there was no evidence of any benefits, and they may be associated with serious physical side effects and could contribute to a worsening of symptoms. Members of the GDG were keen to develop a ‘do not use’ recommendation because of their clinical experience of a number of people actively seeking these interventions as treatments for their social anxiety disorder and a concern that treatment for physical problems could reinforce maladaptive beliefs and worsen symptoms.
The evidence for particular subgroups (that is, people with a fear of public speaking, sweating or blushing) suggested that interventions designed specifically for these fears are not effective. The available evidence supports the use of standard treatments for all forms of social anxiety disorder, so the GDG decided to make no specific recommendations about these subtypes. Similarly, no specific treatments for comorbid disorders were identified that would lead to a modification of existing NICE guidance.
6.13. RECOMMENDATIONS
6.13.1. Treatment principles
- 6.13.1.1.
All interventions for adults with social anxiety disorder should be delivered by competent practitioners. Psychological interventions should be based on the relevant treatment manual(s), which should guide the structure and duration of the intervention. Practitioners should consider using competence frameworks developed from the relevant treatment manual(s) and for all interventions should:
- receive regular, high-quality outcome-informed supervision
- use routine sessional outcome measures (for example, the SPIN or LSAS) and ensure that the person with social anxiety is involved in reviewing the efficacy of the treatment
- engage in monitoring and evaluation of treatment adherence and practitioner competence – for example, by using video and audio tapes, and external audit and scrutiny if appropriate.
- 6.13.1.2.
For people (including young people) with social anxiety disorder who misuse substances, be aware that alcohol or drug misuse is often an attempt to reduce anxiety in social situations and should not preclude treatment for social anxiety disorder. Assess the nature of the substance misuse to determine if it is primarily a consequence of social anxiety disorder and:
- for harmful or dependent alcohol or drug misuse consider referral to a specialist alcohol or drug misuse service20.
6.13.2. Initial treatment options for adults with social anxiety disorder
- 6.13.2.1.
Offer adults with social anxiety disorder individual cognitive behavioural therapy (CBT) that has been specifically developed to treat social anxiety disorder (based on the Clark and Wells model or the Heimberg model; see recommendations 6.13.4.1 and 6.13.4.2).
- 6.13.2.2.
Do not routinely offer group CBT in preference to individual CBT. Although there is evidence that group CBT is more effective than most other interventions, it is less clinically and cost effective than individual CBT.
- 6.13.2.3.
For adults who decline CBT and wish to consider another psychological intervention, offer CBT-based supported self-help (see recommendation 6.13.4.3).
- 6.13.2.4.
For adults who decline cognitive behavioural interventions and express a preference for a pharmacological intervention, discuss their reasons for declining cognitive behavioural interventions and address any concerns.
- 6.13.2.5.
If the person wishes to proceed with a pharmacological intervention, offer a selective serotonin reuptake inhibitor (SSRI) (escitalopram or sertraline). Monitor the person carefully for adverse reactions (see recommendations 6.13.5.1–6.13.5.7).
- 6.13.2.6.
For adults who decline cognitive behavioural and pharmacological interventions, consider short-term psychodynamic psychotherapy that has been specifically developed to treat social anxiety disorder (see recommendation 6.13.4.4). Be aware of the more limited clinical effectiveness and lower cost effectiveness of this intervention compared with CBT, self-help and pharmacological interventions.
6.13.3. Options for adults with no or a partial response to initial treatment
- 6.13.3.1.
For adults whose symptoms of social anxiety disorder have only partially responded to individual CBT after an adequate course of treatment, consider a pharmacological intervention (see recommendation 6.13.2.5) in combination with individual CBT.
- 6.13.3.2.
For adults whose symptoms have only partially responded to an SSRI (escitalopram or sertraline) after 10 to 12 weeks of treatment, offer individual CBT in addition to the SSRI.
- 6.13.3.3.
For adults whose symptoms have not responded to an SSRI (escitalopram or sertraline) or who cannot tolerate the side effects, offer an alternative SSRI (fluvoxamine21 or paroxetine) or a serotonin noradrenaline reuptake inhibitor (SNRI) (venlafaxine), taking into account:
- the tendency of paroxetine and venlafaxine to produce a discontinuation syndrome (which may be reduced by extended-release preparations).
- the risk of suicide and likelihood of toxicity in overdose.
- 6.13.3.4.
For adults whose symptoms have not responded to an alternative SSRI or an SNRI, offer a monoamine oxidase inhibitor (phenelzine22 or moclobemide).
- 6.13.3.5.
Discuss the option of individual CBT with adults whose symptoms have not responded to pharmacological interventions.
6.13.4. Delivering psychological interventions for adults
- 6.13.4.1.
Individual CBT (the Clark and Wells model) for social anxiety disorder should consist of up to 14 sessions of 90 minutes' duration over approximately 4 months and include the following:
- education about social anxiety
- experiential exercises to demonstrate the adverse effects of self-focused attention and safety-seeking behaviours
- video feedback to correct distorted negative self-imagery
- systematic training in externally focused attention
- within-session behavioural experiments to test negative beliefs with linked homework assignments
- discrimination training or rescripting to deal with problematic memories of social trauma
- examination and modification of core beliefs
- modification of problematic pre- and post-event processing
- relapse prevention.
- 6.13.4.2.
Individual CBT (the Heimberg model) for social anxiety disorder should consist of 15 sessions of 60 minutes' duration, and one session of 90 minutes for exposure, over approximately 4 months, and include the following:
- education about social anxiety
- cognitive restructuring
- graduated exposure to feared social situations, both within treatment sessions and as homework
- examination and modification of core beliefs
- relapse prevention.
- 6.13.4.3.
Supported self-help for social anxiety disorder should consist of:
- typically up to nine sessions of supported use of a CBT-based self-help book over 3–4 months
- support to use the materials, either face to face or by telephone, for a total of 3 hours over the course of the treatment.
- 6.13.4.4.
Short-term psychodynamic psychotherapy for social anxiety disorder should consist of typically up to 25–30 sessions of 50 minutes' duration over 6–8 months and include the following:
- education about social anxiety disorder
- establishing a secure positive therapeutic alliance to modify insecure attachments
- a focus on a core conflictual relationship theme associated with social anxiety symptoms
- a focus on shame
- encouraging exposure to feared social situations outside therapy sessions
- support to establish a self-affirming inner dialogue
- help to improve social skills.
6.13.5. Prescribing and monitoring pharmacological interventions in adults
- 6.13.5.1.
Before prescribing a pharmacological intervention for social anxiety disorder, discuss the treatment options and any concerns the person has about taking medication. Explain fully the reasons for prescribing and provide written and verbal information on:
- the likely benefits of different drugs
- the different propensities of each drug for side effects, discontinuation syndromes and drug interactions
- the risk of early activation symptoms with SSRIs and SNRIs, such as increased anxiety, agitation, jitteriness and problems sleeping
- the gradual development, over 2 weeks or more, of the full anxiolytic effect
- the importance of taking medication as prescribed, reporting side effects and discussing any concerns about stopping medication with the prescriber, and the need to continue treatment after remission to avoid relapse.
- 6.13.5.2.
Arrange to see people aged 30 years and older who are not assessed to be at risk of suicide within 1 to 2 weeks of first prescribing SSRIs or SNRIs to:
- discuss any possible side effects and potential interaction with symptoms of social anxiety disorder (for example, increased restlessness or agitation)
- advise and support them to engage in graduated exposure to feared or avoided social situations.
- 6.13.5.3.
After the initial meeting (see recommendation 6.13.5.2), arrange to see the person every 2–4 weeks during the first 3 months of treatment and every month thereafter. Continue to support them to engage in graduated exposure to feared or avoided social situations.
- 6.13.5.4.
For people aged under 30 years who are offered an SSRI or SNRI:
- warn them that these drugs are associated with an increased risk of suicidal thinking and self-harm in a minority of people under 30 and
- see them within 1 week of first prescribing and
- monitor the risk of suicidal thinking and self-harm weekly for the first month23.
- 6.13.5.5.
Arrange to see people who are assessed to be at risk of suicide weekly until there is no indication of increased suicide risk, then every 2–4 weeks during the first 3 months of treatment and every month thereafter. Continue to support them to engage in graduated exposure to feared or avoided social situations.
- 6.13.5.6.
Advise people taking a monoamine oxidase inhibitor of the dietary and pharmacological restrictions concerning the use of these drugs as set out in the British National Formulary (2013)24.
- 6.13.5.7.
For people who develop side effects soon after starting a pharmacological intervention, provide information and consider one of the following strategies:
- monitoring the person's symptoms closely (if the side effects are mild and acceptable to the person)
- reducing the dose of the drug
- stopping the drug and offering either an alternative drug or individual CBT, according to the person's preference25.
- 6.13.5.8.
If the person's symptoms of social anxiety disorder have responded well to a pharmacological intervention in the first 3 months, continue it for at least a further 6 months.
- 6.13.5.9.
When stopping a pharmacological intervention, reduce the dose of the drug gradually. If symptoms reappear after the dose is lowered or the drug is stopped, consider increasing the dose, reintroducing the drug or offering individual CBT.
6.13.6. Interventions that are not recommended to treat social anxiety disorder
- 6.13.6.1.
Do not routinely offer anticonvulsants, tricyclic antidepressants, benzodiazepines or antipsychotic medication to treat social anxiety disorder in adults.
- 6.13.6.2.
Do not routinely offer mindfulness-based interventions26 or supportive therapy to treat social anxiety disorder.
- 6.13.6.3.
Do not offer St John's wort or other over-the-counter medications and preparations for anxiety to treat social anxiety disorder. Explain the potential interactions with other prescribed and over-the-counter medications and the lack of evidence to support their safe use.
- 6.13.6.4.
Do not offer botulinum toxin to treat hyperhidrosis (excessive sweating) in people with social anxiety disorder. This is because there is no good-quality evidence showing benefit from botulinum toxin in the treatment of social anxiety disorder and it may be harmful.
- 6.13.6.5.
Do not offer endoscopic thoracic sympathectomy to treat hyperhidrosis or facial blushing in people with social anxiety disorder. This is because there is no good-quality evidence showing benefit from endoscopic thoracic sympathectomy in the treatment of social anxiety disorder and it may be harmful.
6.13.7. Research recommendations
- 6.13.7.1.
What is the clinical and cost effectiveness of combined psychological and pharmacological interventions compared with either intervention alone in the treatment of adults with social anxiety disorder? (See Appendix 9 for further details.)
- 6.13.7.2.
What is the clinical and cost effectiveness of additional psychological and pharmacological interventions in the treatment of adults with social anxiety disorder who have not recovered when treated with individual CBT?
Footnotes
- 17
Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
- 18
NICE (2011).
- 19
- 20
This recommendation also appears in Chapter 7 regarding interventions for children and young people.
- 21
At the time of publication, fluvoxamine did not have a UK marketing authorisation for use in adults with social anxiety disorder. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Good Practice in Prescribing and Managing Medicines and Devices (2013) for further information.
- 22
At the time of publication, phenelzine did not have a UK marketing authorisation for use in adults with social anxiety disorder. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Good Practice in Prescribing and Managing Medicines and Devices (2013) for further information.
- 23
This recommendation is adapted from Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults (NICE clinical guideline 113; NICE, 2011b).
- 24
- 25
This recommendation is adapted from Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults (NICE clinical guideline 113; NICE, 2011b).
- 26
This includes mindfulness-based stress reduction and mindfulness-based cognitive therapy.
- INTRODUCTION
- CURRENT PRACTICE
- DEFINITIONS AND AIMS OF INTERVENTIONS
- CLINICAL REVIEW PROTOCOL
- OVERVIEW OF STUDIES CONSIDERED AND CLINICAL EVIDENCE
- PHARMACOLOGICAL INTERVENTIONS
- PSYCHOLOGICAL INTERVENTIONS
- COMBINED PSYCHOLOGICAL AND PHARMACOLOGICAL INTERVENTIONS
- SPECIFIC SUBGROUPS
- HEALTH ECONOMIC EVIDENCE
- OVERALL CLINICAL SUMMARY
- FROM EVIDENCE TO RECOMMENDATIONS
- RECOMMENDATIONS
- INTERVENTIONS FOR ADULTS - Social Anxiety DisorderINTERVENTIONS FOR ADULTS - Social Anxiety Disorder
Your browsing activity is empty.
Activity recording is turned off.
See more...
