India could soon allow ‘game-changing’ weight-loss drug tirzepatide: How it works, its side effects
Obesity drugs are also not one-time miracle solutions for weight loss. But in recent years, the development of such drugs globally has been game-changing.
 Obesity drugs are also not one-time miracle solutions for weight loss — data from trials indicate that these drugs need to continue to be taken for their weight loss and other effects to last. (Via Pixabay)
Obesity drugs are also not one-time miracle solutions for weight loss — data from trials indicate that these drugs need to continue to be taken for their weight loss and other effects to last. (Via Pixabay)The development of various weight loss drugs has been a game changer for obesity treatment in recent years, especially in the US and Europe. But these drugs are yet to be commercially available in India, with pending regulatory clearances and high demand abroad delaying their arrival in the country.
But this might soon change. Last week, in a first, an expert committee of India’s drug regulator gave the green light to the drug tirzepatide. Following a review of this recommendation, the drug will be given final approval by the regulator, allowing its manufacturer, Eli Lilly, to launch the product in the Indian market.
Diabetes drug for weight loss
In 2017, the US Food and Drugs Administration (FDA) approved Danish pharma giant Novo Nordisk’s Ozempic, with the active ingredient semaglutide, to manage type 2 diabetes. Soon, doctors in the US saw an interesting side-effect — weight loss.
They started prescribing Ozempic off-label (the practice of prescribing a drug for a different purpose than what has been approved) to treat obesity. A social media frenzy followed, with influencers flooding TikTok and Instagram with posts about their dramatic weight loss transformations, all courtesy Ozempic.
This made Novo Nordisk explore semaglutide as a weight loss drug for people without diabetes. In 2021, the company released Wegovy, a semaglutide injection, as an FDA-approved obesity treatment. The key difference between Ozempic and Wegovy: the maximum approved dose of semaglutide is slightly higher with Wegovy than Ozempic. Currently, there is a global shortage of both drugs amid soaring demand.

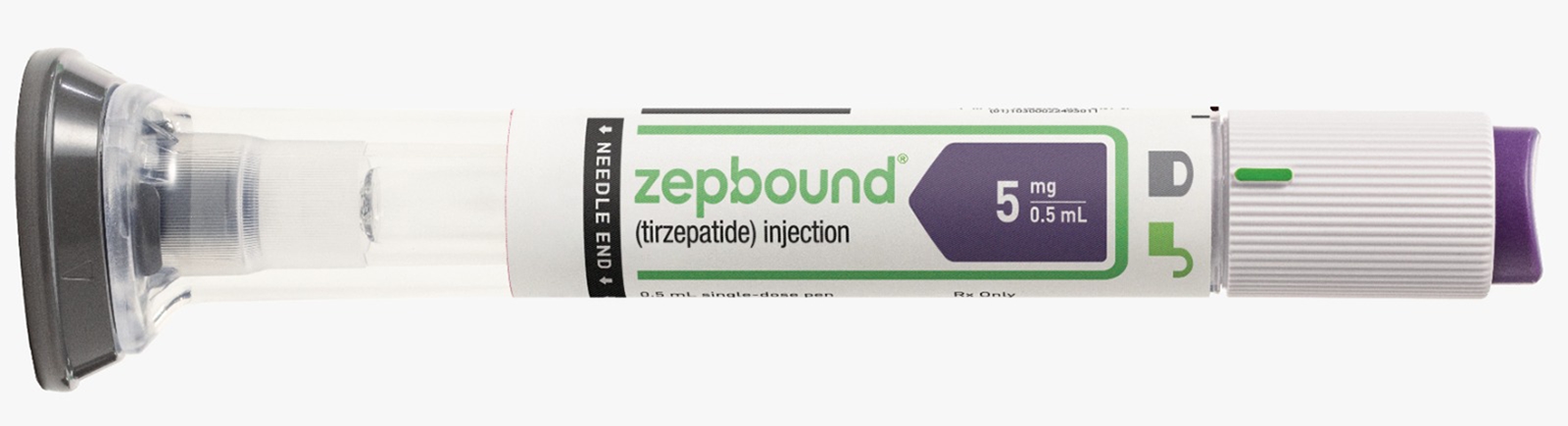 Weight loss drug tirzepatide, marketed by US pharma giant Eli Lilly as Zepbound, is on the cusp of getting regulatory approval in India.
Weight loss drug tirzepatide, marketed by US pharma giant Eli Lilly as Zepbound, is on the cusp of getting regulatory approval in India.
In November 2023, Eli Lilly, another US pharma major, got FDA approval for the drug Zepbound to treat obesity. This came just over a year after its type 2 diabetes medication, Mounjaro, was launched. Like Ozempic, Mounjaro too led to weight loss among users, and began to see rampant off-label use. Zepbound and Mounjaro contain tirzepatide as the active ingredient. Both face shortages in the global market.
Semaglutide vs tirzepatide
The FDA has approved Wegovy (semaglutide) and Zepbound (tirzepatide) for chronic weight management in adults. These drugs can be prescribed to those who are obese (with a body mass index of over 30), or overweight (with a BMI between 27 and 30), and have at least one other health condition related to their weight (such as high blood pressure, high cholesterol, or type 2 diabetes).
Both are administered as under-the-skin injections, and are intended to be used alongside a reduced-calorie diet and increased physical activity. The dosage is increased gradually, reaching a maximum dosage of 2.4 mg for semaglutide and 15 mg for tirzepatide. This does not, however, mean that the latter is ‘stronger’ than the former.

Semaglutide and tirzepatide are polypeptides, small proteins that boost the levels of naturally-occurring hormones in the body, including that of glucagon-like-peptide 1 (GLP-1), which control weight through the brain and digestive tract.
Higher GLP-1 levels, released in the gut, spark a reaction by stimulating neurons that alter gut function, leading to a sense of fullness. This process also taps into a brain mechanism that lights up neural pathways, triggering the sensation of satiety — the feeling of being satisfied and having had enough to eat.
They also help manage glucose levels, making them an effective treatment for diabetes.
Semaglutide only targets GLP-1 receptors. On the other hand, tirzepatide also boosts a second hormone: glucose-dependent insulinotropic polypeptide (GIP). The GIP also regulates weight through receptors in brain and fat cells. Eli Lilly claims that the combined action of GLP-1 and GIP enhance each other’s effects.
Promising global trials
Global trials for Zepbound have yielded promising results. The phase three trials involved 2,539 participants, each randomised to receive either a placebo or one of three doses of tirzepatide: 5 mg, 10 mg, or 15 mg.
Over the course of 72 weeks, those on the 5 mg dose lost 15% of their body weight on average, while the 10 mg group shed 19.5%. The 15 mg group achieved a remarkable 20.9% weight reduction — this translates to someone weighing 75 kg shedding more than 15 kg. About 91% of individuals on the 15 mg dose achieved a weight loss of at least 5%. In stark contrast, the placebo group had an average weight reduction of only 3.1%.
This sustained weight reduction was accompanied by improvements in all prespecified cardiometabolic measures.
Zepbound got regulatory clearance in India based on data from these trials, in which Indians took part as well. But the aforementioned expert committee has imposed a crucial rider — the company will have to conduct a phase IV, post-marketing surveillance trial to scrutinise side effects that might have been missed in previous trials, and the drug’s efficacy among India’s diverse population.
Some side effects of the drug
According to the company, Zepbound’s most common side effects include nausea, diarrhoea, vomiting, constipation, abdominal pain, indigestion, injection-site reactions, fatigue, allergic reactions, belching, hair loss, and heartburn.
Eli Lilly specifically highlights the risk of thyroid tumours, including thyroid cancer. “Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath,” the manufacturer says. Individuals cannot use Zepbound if they, or any family members, have ever had medullary thyroid carcinoma (MTC), a type of thyroid cancer, or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), a rare, inherited disorder that affects the endocrine glands.
Zepbound is a prescription medicine. It cannot — and should not — be used for cosmetic weight loss.
Weight back if drug is stopped
Obesity drugs are also not one-time miracle solutions for weight loss — data from trials indicate that these drugs need to continue to be taken for their weight loss and other effects to last.
In Wegovy’s STEP 1 extension trial with 327 participants, those using Wegovy for 68 weeks achieved a remarkable average weight loss of 17.3%, compared to just 2.0% for those on a placebo. But after stopping the medication, by week 120, Wegovy users had regained most of their weight — effectively seeing an average weight loss of only 5.6% compared to 0.1% for the placebo group. Improvements in heart and metabolic health seen during the treatment period also tended to revert to baseline levels once the treatment was stopped.
At the end of the day, obesity is a complex, chronic, and progressive disease which has to be managed throughout one’s life.
More Explained
EXPRESS OPINION
Aug 22: Latest News
- 01
- 02
- 03
- 04
- 05







































