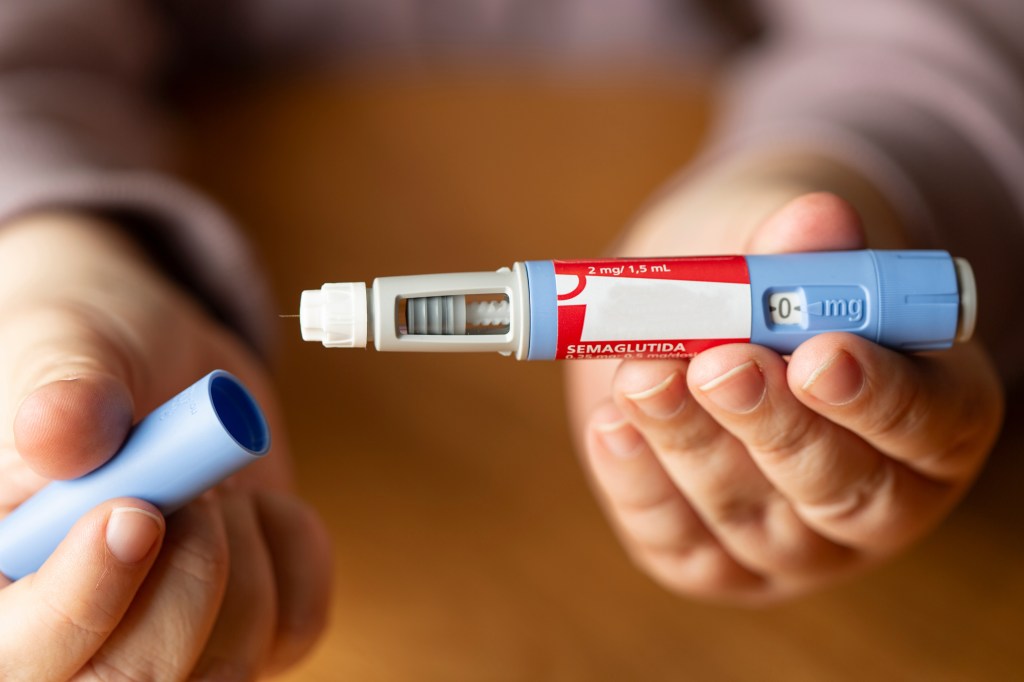
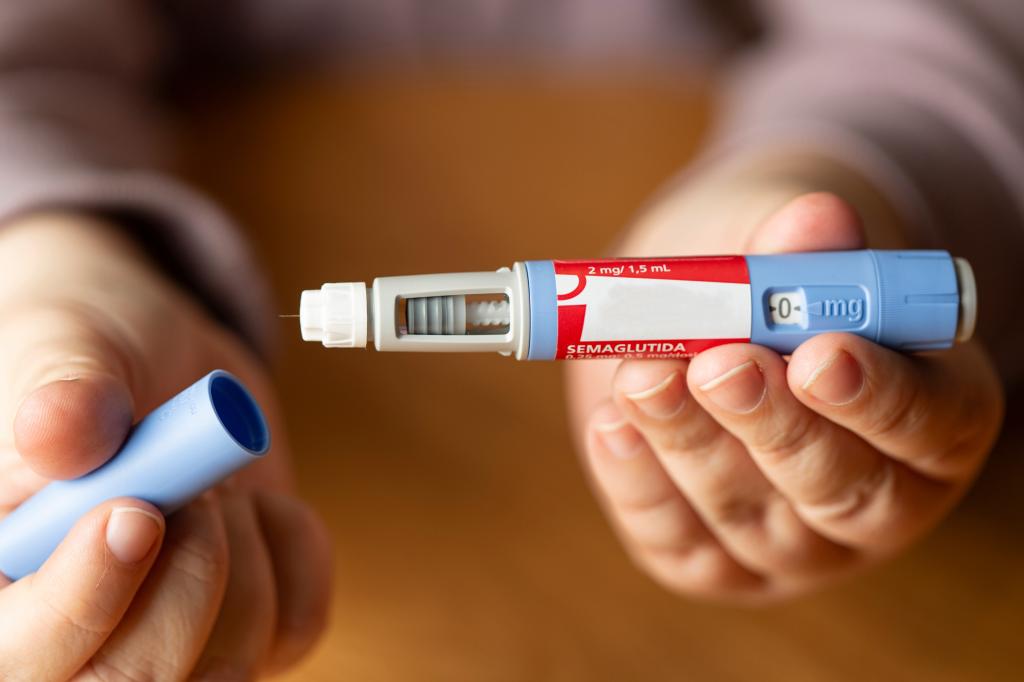
Telehealth provider Hims & Hers announced Monday it will sell a compound version of the popular weight-loss drugs Ozempic and Wegovy.
The news comes as demand for Wegovy and other GLP-1 drugs has skyrocketed, leaving drugmakers, like Novo Norodisk and Eli Lily, struggling with supply shortages that have frequently left desperate patients without an alternative.
In an interview with CNBC, Andrew Dudum, CEO of Hims & Hers, stated that the company has partnered with one of the largest manufacturers of generics in the US to eliminate supply issues.
“We have a certain degree of exclusivity with that facility that will guarantee our consumers consistent volume and supply,” he explained to the outlet.
First approved to treat patients with Type 2 diabetes, these GLP-1 weight loss drugs have quickly gained traction among celebrities and mere mortals alike seeking to slim down. Wegovy is currently the only brand of semaglutide (essentially a hormone used to improve blood sugar) approved to treat obesity.
The Hims version of the GLP-1 drug semaglutide will be prescribed by physicians through the Hims & Hers digital platform.
With price points starting at $199 per month for injections and $79 for oral kits, Hims & Hers medications offer a much more affordable alternative to brand-name drugs like Ozempic and Wegovy which routinely demand prices upwards of $1,300 per package.
The Post reports these prices may border on extortion, with recent research suggesting Ozempic can be manufactured for just $5.
In addition to the high cost, these drugs have been known to wreak havoc on health, as some users reported serious adverse side effects, such as stomach paralysis or intestinal blockage. Will these new, cheaper compound versions, which are less regulated and inherently more risky lead to further damage?
What is compounding?
Compounded drugs are made when a pharmacist combines, mixes or alters a drug formula in order to tailor it to a patient. Compounding medications are commonly used when a patient is allergic to an ingredient in a commercially available product or when the demand for a drug surpasses supply.
Unlike medications produced by large manufacturers, compound medications like the semaglutide offered by Hims & Hers are not approved by the FDA.
As Dr. Michael O. McKinney, a weight-loss physician at Healthy Outlook explains, “The FDA approval process applies to mass-produced medications and involves rigorous testing to ensure safety, efficacy and quality,” a process of precaution that compounded drugs are not subjected to.
Is it safe?
While the new availability may seem like a boon to a high demand market, there are some serious risks associated with the manufacturing and consumption of compounded drugs.
In January, the FDA issued a statement warning individuals against buying GLP-1s from compounding pharmacies, in part, because the drugs are not evaluated for safety or efficacy due to their individual nature.
Dr. Kubanych Takyrbashev, Health & Wellness Advisor at NAO, explains, “Unlike FDA-approved drugs that must meet strict standards for how they are absorbed and utilized by the body, compounded medications can vary significantly. This can mean that one batch of compounded semaglutide might be absorbed differently or have a different potency than another, leading to unpredictable therapeutic outcomes.”
In addition to unpredictable outcomes, compounded medications pose the risk of unknown side effects and adverse interactions. Possible problems with sterility and cleanliness in the manufacturing facility are also cause for concern.
Perhaps the most serious issue associated with compounded drugs is the lack of accountability. With FDA-approved medications, users can report problems to the FDA, with compounded drugs there is no such recourse.