Deja-flu: Biden Administration nearing deal to bankroll Moderna's MRNA vaccine for H5N1 influenza amid fears virus could cause pandemic
- READ MORE: Four more pet cats die from bird flu - raising fears of a spillover
The Biden Administration is on the verge of a deal to bankroll Moderna's development of a vaccine against bird flu, reports suggest.
In a similar operation that sped up the production of Covid vaccines, the US government is looking to fund a late-stage trial of the mRNA shot, targeting the H5N1 strain that is currently circulating in the US.
It is unclear how much it is costing but it is likely tens of millions of dollars - in return for the US getting priority of doses.
The current bird flu outbreak - one of the worst in recent history - has seen the virus detected on chicken farms in 48 states and in dairy cow herds across nine states in the US.
The US government looking to fund a late-stage trial of Moderna's mRNA pandemic bird flu vaccine
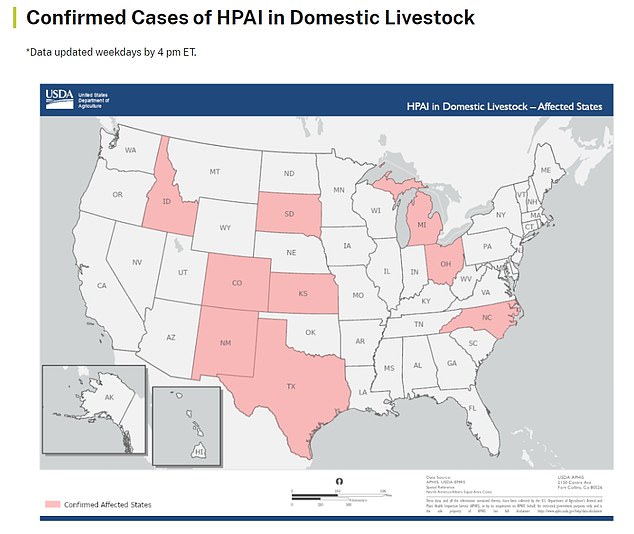
The above map shows the states that have reported bird flu infections in dairy herds
Two people have become infected, as well as detections in milk and beef samples, leading to fears it could start spreading more easily among people.
There is particular concern because H5N1 has a death rate of about 50 percent, compared to Covid which was around one percent.
The deal incentivizes pharmaceutical companies to generate a vaccine, while offsetting their risk of losses.
If the vaccine does not work, the government will not take on the doses, and will be reliant on the existing vaccines it has stockpiled but is looking to bolster.
The mRNA bird flu vaccine is based on the same technology as Pfizer's and Moderna's Covid vaccines.
Traditional vaccines tend to be egg-based. A selected virus is injected into a hen's eggs where it incubates and replicates for a few days in the same way it would in a human.
Scientists harvest liquid inside the egg that contains the virus and inactivate it so it can no longer cause disease, purify it, and leave behind the crucial antigen that prompts an immune response in the event of infection.
But this can take up to six months. MRNA vaccines, on the other hand, can be made within hours of researchers sequencing a new viral strain.
Moderna and Pfizer's mRNA vaccines direct cells in the body to make proteins to prevent or fight bird flu using messenger ribonucleic acid, a molecule which is key to the functioning of the body's cells.
The shot would deliver small particles containing the instructions to make proteins found on the surface of the bird flu virus into cells.
Traditional vaccines tend to be egg-based. A selected virus is injected into a hen's eggs where it incubates and replicates for a few days in the same way it would in a human
The cells then begin manufacturing these proteins, which sets off an immune response.
Researchers have tested the vaccine on mice and ferrets and have observed a strong antibody and T-cell response in the animals.
The vaccine will need to produce successful results in the late stage trial. After that, it will need to enter clinical trials on humans, and be approved by the FDA if those trials go well.
The US Agriculture Department has also proposed allowing farmers to bulk test the milk of their dairy cows for bird flu instead of testing milk from individual cows before getting approval to ship the animals across sates.
A pilot program for bulk testing milk could begin in June for farmers who choose to participate, according to documents USDA sent to industry officials this week obtained by Reuters.
The US, Canada and Europe have also been in active talks with pharmaceutical companies CSL Seqirus and GSK to acquire or manufacture H5N1 bird flu vaccines, which could be used to protect at-risk poultry and dairy workers, veterinarians and lab technicians.
The US has a stockpile of bird flu vaccines matched with the strain currently circulating, as well as antivirals that could be used to treat human infections.
For a major epidemic or a pandemic, the US would have to scale up considerably.
The government is also in 'active conversations' with mRNA vaccine makers Pfizer and Moderna on a potential vaccine for humans.
There are also talks between the government and Pfizer over financial support for the development of its mRNA vaccine targeting the H5 family of viruses.
Like Moderna, Pfizer also played a key part in generating mRNA vaccines for the Covid pandemic.
Last week, US health officials said the government was proceeding with plans to fill 4.8 million vials from its existing stock of protein-based bird flu vaccines.
Existing animal vaccines for bird flu are currently not used on a wide scale on bird farms because it lessens the ability to monitor outbreaks of the disease.
However, there are some readily available antiviral drugs that can treat severe flu, like oseltamivir.
Hopefully these would work on any pandemic bird flu virus, but viruses can become resistant.
Bird flu has fueled concerns as the disease is increasingly spreading to mammals, with the first-ever outbreaks detected dairy cows in the US, raising concerns about it spreading to humans through the nation's milk supply.